I forget whether this question came up in our discussion of the Scholz paper (paper #1), but 2-norbonyl cation was crystallized with Al2Br7 anion. The authors described this anion as “weakly coordinating,”
Our interest in binding challenging ions such as the [CX3]+ (X = Cl, Br, or I) cations (35–37) to weakly coordinating anions (38, 39) led us to exploit the ability of soft bromoaluminate anions, such as [Al2Br7]–, to stabilize, for example, the [C(CH3)3]+ carbocation in the solid state (40).
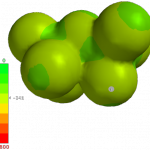
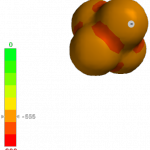
The unusual properties of this anion leap out from a comparison of potential maps of Al2Br7 anion (left) with BF4 anion (right) (EDF2/6-31g* made with iSpartan). Potentials on the former map never get more negative than approx. -340 kJ/mol, while those on the latter reach much more negative values (the extreme occurs at -615 kJ/mol in the orange-red bands between F’s).

Recent Comments