The authors of paper #2 reported on the behavior of a chiral dienophile in Diels-Alder reactions. For reasons that I will discuss on another day, they wanted to conduct their reactions at the lowest possible temperature and so a Lewis acid catalyst was required. They investigated several catalysts based on B and Al compounds and this raised some questions during our discussion last Monday.
A comment that was made in class was that BCl3 was a “hard” Lewis acid and BI3 was probably a “softer” Lewis acid. The theory of hard and soft Lewis acids and bases (HSAB) is taken up in inorganic chemistry so I won’t go into it much here, but I would like to acquaint you with an interpretation of HSAB that is based on Frontier MO theory. Namely,
- Hard acids (and bases) tend to have smaller atoms with larger atomic charges. The “density” of charge (which you can think of as charge/volume) is high in a hard acid/base. One way to quantify this feature is to examine the potential maps of different acids. “Dense” charges produce larger potentials so you can make these connections: large potential –> dense charge –> hard acid (or base). Hard acids are attracted to hard bases by electrostatic forces.
- Soft acids tend to be molecules in which the LUMO (lowest energy unoccupied MO) is lower in energy and better able to accept electrons. Softer bases tend to be molecules in which the HOMO (highest energy occupied MO) is higher in energy. Soft acids bind more strongly to soft bases because the energy gap between LUMO(acid) and HOMO(base) is small.
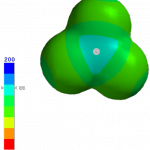
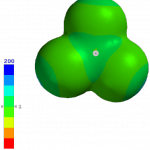
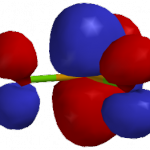
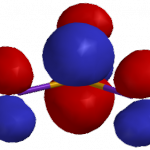
You can see how both effects play out for BCl3 and BI3 using molecular models. The following images were generated using iSpartan (EDF2/6-31G*):
Potential maps, -200 to +200 kJ/mol. BCl3 (left) has a much more positive potential near B (+86 kJ/mol). The potential near B in BI3 is virtually zero, i.e., not positive at all, so BCl3 is the harder acid.
LUMO surfaces & energies. These orbitals look similar for BCl3 (left) and BI3 (right). The central atom (B) makes a large p-type contribution, but there are also smaller antibonding p-type contributions from the halogens. Most significantly, BI3 has the lower energy LUMO (-2.65 eV) and BCl3 has the higher energy LUMO (-1.95 eV). So BI3 is the softer acid.
Because the Frontier MO definitions link hard and soft to different molecular properties, you might wonder, “is it possible to have an acid (or base) with a big charge AND a suitable frontier MO energy? In other words, can a reagent be both “hard” and “soft” simultaneously.
Hmmm. That’s a good question.

Recent Comments