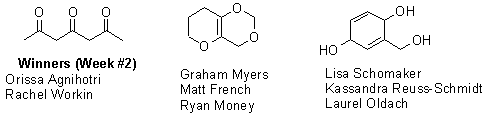
The final week of the C7 H10 O3 contest brought in two entries. Gabe Holt and Patrick Fink had submitted an entry during week #1, but all of the other participants were brand new, bringing the total number of participating students to 19 (one-third of the class).
Unfortunately, neither team could come up with an isomer that had been described in the scientific literature so we don’t really have a “winner” in the usual sense this week. However, in the event of a tie, the judges (me) have decided that the runner-ups will split the usual prizes, so everyone will go home with half of a milk chocolate truffle pig. Enjoy. Here are the structures that were submitted this week:
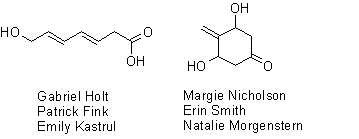
And since you are probably dying to know what the best-studied isomers look like,