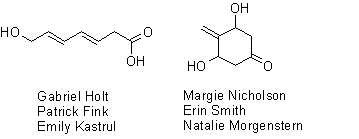
The final week of the C7 H10 O3 contest brought in two entries. Gabe Holt and Patrick Fink had submitted an entry during week #1, but all of the other participants were brand new, bringing the total number of participating students to 19 (one-third of the class).
Unfortunately, neither team could come up with an isomer that had been described in the scientific literature so we don’t really have a “winner” in the usual sense this week. However, in the event of a tie, the judges (me) have decided that the runner-ups will split the usual prizes, so everyone will go home with half of a milk chocolate truffle pig. Enjoy. Here are the structures that were submitted this week:
And since you are probably dying to know what the best-studied isomers look like,
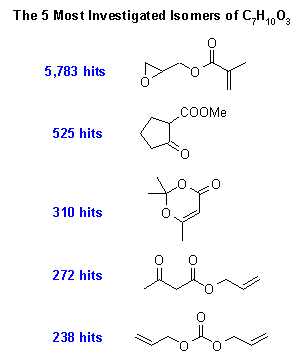
I have listed the Top 5 in the following chart. The #1 compound
contains a strained epoxide ring (we will study the chemistry of this
group later in the semester) and an unsaturated ester (this group will
be described next semester in 202). Pat speculates that this compound
is used to make polymers, or perhaps adhesives. Interestingly, there is
a huge drop-off of interest in going from #1 (5783 hits) to #2 (525
hits), but #2 looks like a real winner: the ketone-ester combination is
commonly encountered and it will be the subject of a lecture or two
next semester. By the way, Pat submitted his own guess on the sly
during week #1. It didn’t quite crack the Top 5 list, but he just
missed by a hair: it was #6 and had 218 hits. So next time, bribe Pat
for some hints and take home the chocolate!

Please…as if we, oh novices in O chem, could have decided that a strained epoxide ring was plausible. If I tried to build that, it would break my model set.
Well, I can’t argue with any of that. I certainly wouldn’t want you to break your models this early in the term.
FYI – I had no idea what any of the possible answers were going to be when I set up the contest. I would never have guessed most of the top 5 structures even though I can see the “logic” behind some of them now that I know what they are (and neither could my in-house expert, who came up with only #6 as his best guess).
The point of the contest was partly to see how little we have really learned as organic chemists, but mainly to work with classmates and have some fun.