A question on today’s conference problem set asked you to predict how the energies of the pi and pi* orbitals of a typical alkene would respond to twisting the alkene.
Valence bond fans: The VB model doesn’t contain “pi” or “pi*” orbitals, but it correctly predicts that twisting the alkene destroys p-p overlap, i.e., destroys the pi bond and destabilizes the molecule.
The following picture shows the answer to the conference problem (click for larger image). The planar alkene is shown on the left and the fully twisted alkene on the right. The pi MO (bottom) and pi* MO (top) energies of the planar alkene are very different (vertical axis is MO energy). As we twist the alkene, the two MO energies converge.
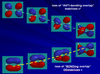
To make this prediction you need to visualize each MO as being constructed from two 2p orbitals. If the overlap between these 2p “pieces” is “bonding” (no node + orbitals close together), as in the pi MO, it stabilizes the MO. Twisting reduces the overlap and destabilizes the pi MO. When the overlap disappears completely, the pi MO energy becomes equal to that of a 2p orbital.
The opposite behavior is seen when overlap between the 2p orbitals is “ANTIbonding” (node + orbitals close together). This kind of overlap destabilizes the MO and that’s why the pi* MO is higher in energy. Twisting the alkene stabilizes the pi* MO because the antibonding overlap is reduced. When the antibonding overlap disappears completely, the pi* MO energy equals that of the pi MO.
