Spartan can produce a number of “job failed” messages. The most frequent reason for failure is “maximum number of optimization cycles was reached.” I’ll explain what this message means, why it is so common, and what you can do about it.
You are currently browsing the monthly archive for February 2015.
Most papers that include molecular models publish the atomic coordinates and total energies in the supporting information. The list of coordinates can be easily downloaded, saved on your computer, and converted into a Spartan’14 model by following these steps:
The authors of paper #2 reported on the behavior of a chiral dienophile in Diels-Alder reactions. For reasons that I will discuss on another day, they wanted to conduct their reactions at the lowest possible temperature and so a Lewis acid catalyst was required. They investigated several catalysts based on B and Al compounds and this raised some questions during our discussion last Monday.
Today’s class presented two formalisms that organic chemists use for connecting rates, rate constants, and the shapes of PE surfaces:
- Arrhenius equation (old-school but still useful and still widely used; go here for more background on collisions & kinetics)
- transition state theory (more obvious connection to thermodynamic quantities, but web pages say this is not useful when barriers get very small)
I forget whether this question came up in our discussion of the Scholz paper (paper #1), but 2-norbonyl cation was crystallized with Al2Br7 anion. The authors described this anion as “weakly coordinating,”
Our interest in binding challenging ions such as the [CX3]+ (X = Cl, Br, or I) cations (35–37) to weakly coordinating anions (38, 39) led us to exploit the ability of soft bromoaluminate anions, such as [Al2Br7]–, to stabilize, for example, the [C(CH3)3]+ carbocation in the solid state (40).
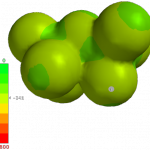
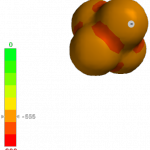
The unusual properties of this anion leap out from a comparison of potential maps of Al2Br7 anion (left) with BF4 anion (right) (EDF2/6-31g* made with iSpartan). Potentials on the former map never get more negative than approx. -340 kJ/mol, while those on the latter reach much more negative values (the extreme occurs at -615 kJ/mol in the orange-red bands between F’s).

Recent Comments