Cellular migration is an essential feature of the development of tissues, organs, and overall facial morphogenesis. SPECC1L encodes a protein that functionally interacts with actin and microtubules, two key components of the cell cytoskeleton. Mutations in SPECC1L observed in humans, zebrafish, and Drosophila, reveal that SPECC1L plays an essential role in the development of a “face”, through the closure of the neural tube, and its role in cranial neural crest cell delamination. Cranial neural crest cell (CNCC) delamination describes the migration of CNCCs from the embryonic neural folds to the pharyngeal arches, thereby helping to form key features of the embryo face.
This delamination implies the role of SPECC1L in cellular contractility and migration. Non-muscle myosin II plays a critical role in the development of cellular protrusions, known as lamellipodia, necessary to the process of cell migrations. Focal adhesions form in the lamellipodia, and mature in the lamella, which is characterized by thicker bundles of actin and slower retrograde flow. These focal adhesions are critical to the ability of the cell to form attachments to the extracellular matrix (the substrate on which it crawls).
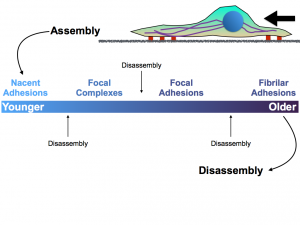
Figure 1. The above figure depicts the different types of attachments that the cytoskeleton and cell can make to the extracellular matrix. While disassembly can occur at any point within the maturation of the nascent adhesions in the lamella, the formation of focal adhesions and fibrillar adhesions is critical to pulling the cell forward.
The role of non-muscle myosin II in the formation of these cellular adhesions to the extracellular matrix suggests that it may be affected by the depletion of SPECC1L. Students in both biology and physics will collaborate on this research project to ultimately develop a greater understanding of the association between non-muscle myosin II and SPECC1L.
References:
- L. Gfrerer, V. Shubinets, T. Hoyos, Y. Kong, C. Nguyen, P. Pietschmann, C.C. Morton, R. L. Maas, E.C. Liao. Functional analysis of SPECC1L in craniofacial development and oblique facial cleft pathogenesis. Plast. Reconstr. Surg., 134 (2014), pp. 748-759
- Vicente-Manzanares, M., Ma, X., Adelstein, R. S. & Horwitz, A. R.Non-muscle myosin II takes centre stage in cell adhesion and migration. Nature Rev. Mol. Cell Biol. 10, 778– 790 (2009).
- Saadi I, Alkuraya FS, Gisselbrecht SS, Goessling W, Cavallesco R, Turbe-Doan A, et al. Deficiency of the cytoskeletal protein SPECC1L leads to oblique facial clefting. Am J Hum Genet (2011) 89(1):44–55. doi:10.1016/j.ajhg.2011.05.023
- Wilson, N. R., Olm-Shipman, A. J., Acevedo, D. S., Palaniyandi, K., Hall, E. G., Kosa, E., et al. (2016). SPECC1L deficiency results in increased adherens junction stability and reduced cranial neural crest cell delamination. Sci. Rep. 6:17735. doi: 10.1038/srep17735
One thought on “SPECC1L”
Comments are closed.