Our team will use a physical approach to investigate the role of SPECC1L (Split Discs) in cellular contractility by quantifying force expression through Traction Force Microscopy (TFM).
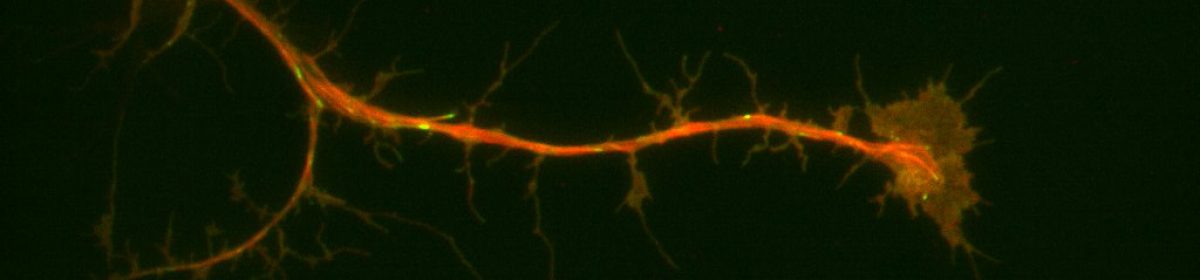
In TFM, cells are adhered to compliant gel matrices with fluorescent beads. The traction forces are found as shown in Fig. 1
Figure 1: Measurement of cellular contraction in cells (Jacobs et al., 2012)

The movement of the fluorescent beads is tracked using the microscope, and along with the known physical characteristics of the substrate such as thickness and stiffness, we can extrapolate the force vectors between the cell and gel matrix.
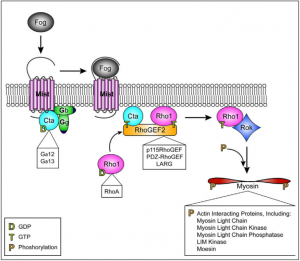
We are using gene inhibition using dsRNA induced gene silencing (RNAi) to target proteins such as Spaghetti Squash (Sqh), Myosin Binding Subunit (Mbs), and our protein of interest, SPECC1L. We will compare the force expression of Split Discs against that of Sqh depleted cells, which will cause the inactivation of non-muscle Myosin II (NMII) and hypocontractile cells, as well as Mbs depleted cells, which will result in the absence of dephosphorylation of RLC, causing an open NMII and hypercontractility (Abidi, 2016).
References:
Abrar A. Abidi. Quantifying cellular mechanotransduction in morphogenesis and cancer. Reed College, 2016.
Christopher R. Jacobs, Hayden Huang, and Ronald Y Kwon. Introduction to Cell Mechanics and Mechanobiology. Garland Science, 2012.