‘Polarizing’ Adventures: Microtubules Lead the Way
-By Nicole Chan
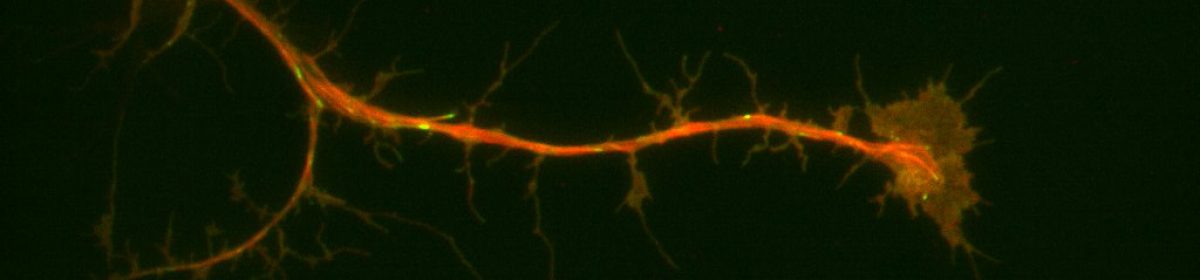
Cell migration depends on the dynamics of the actin and microtubule cytoskeleton. Classically, the actin cytoskeleton is the “engine” of migration, driving cell protrusion. The microtubule cytoskeleton has long been hypothesized to play the role of “compass”, dictating the direction of migration. Another key element to cell migration is focal adhesions, plague-like structures made up of some 120 different proteins that connect the cytoskeleton to the extracellular matrix (ECM). To put it all together briefly, protrusion from the actin cytoskeleton lays the groundwork for the formation of focal adhesions which mature and strengthen the more the cell physically pulls on them. Meanwhile, microtubules direct where protrusions occur and effectively steer the cell. Focal adhesion expression and dynamics also correlate strongly with metastasis in human epithelial cancers which is why studying them has important ramifications for human health. A recent publication on focal adhesions from the Applewhite lab has found that disruption of microtubule polarity (via depletion of the major actin-microtubule cross-linking factor known as Short stop of Shot) results in faster focal adhesion turnover. This ultimately results in faster cell migration (Zhao, AJ., Montes-Liang J., et al, 2022). If disruption of microtubule alignment with actin and focal adhesions led to faster focal adhesion turnover, we wondered what would happen to focal adhesion dynamics if microtubule polarity was forced in the direction of migration. Drosophila cells are a somewhat naive system to test these ideas as they lack an active centrosome during interphase. By expressing a constitutively active Polo Kinase construct, we can activate the centrosome, leading to the formation of a microtubule organization center which then can force the microtubules, putatively in the direction of migration. Results from Summer 2023 research, indicate a presence of a centriole-like structure that organizes the microtubules. It is our hypothesis that this forced polarity will result in cell-matrix adhesions that will last longer, but it is not known how this impacts cell migration. The primary question driving this project is how Drosophila cell-matrix adhesion dynamics behave in cells with or without active centrosomes. However, due to the short timeline, I will be aiming to quantify cell-matrix adhesion dynamics in cells with and without centrosomes.
Zhao, A. J., Montes-Laing, J., Perry, W. M. G., Shiratori, M., Merfeld, E., Rogers, S. L., & Applewhite, D. A. (2022). The Drosophila spectraplakin Short stop regulates focal adhesion dynamics by cross-linking microtubules and actin. Molecular biology of the cell, 33(5), ar19. https://doi.org/10.1091/mbc.E21-09-0434