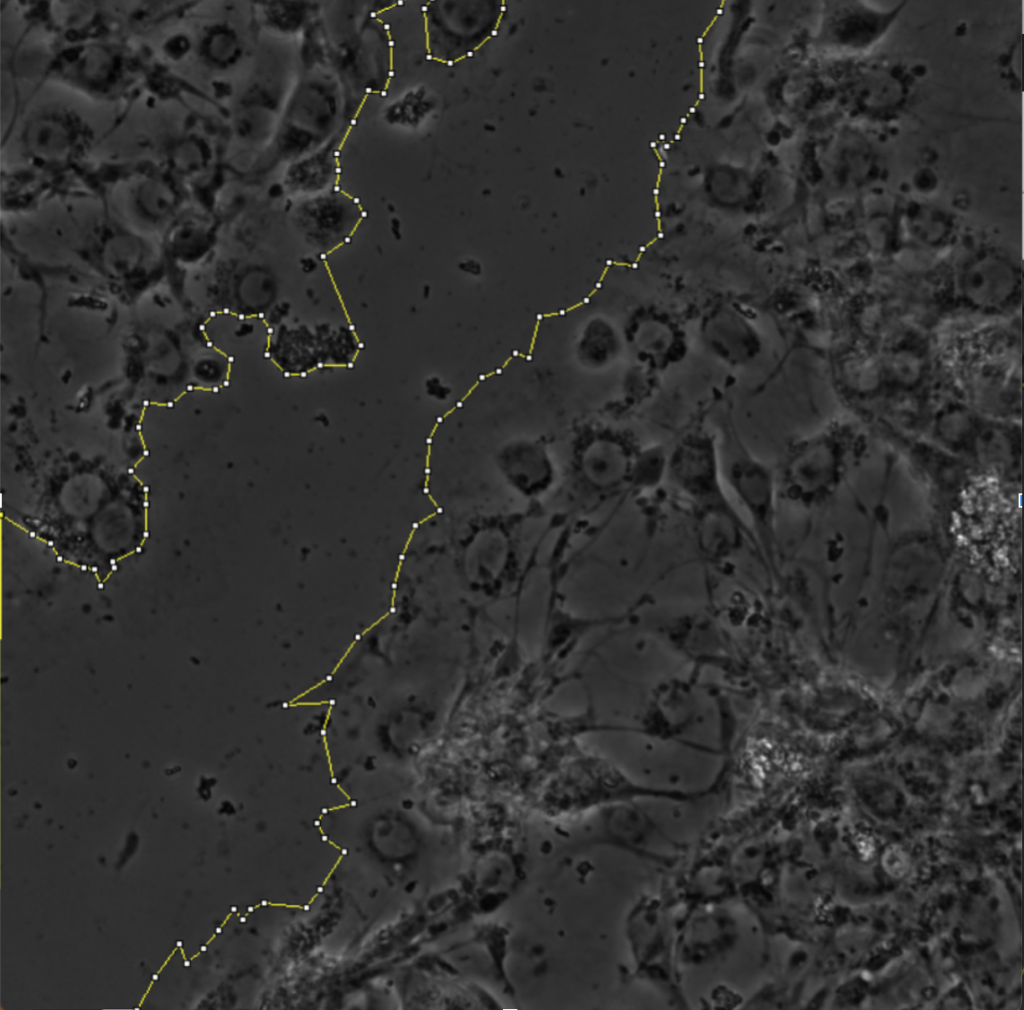
The scratch-wound assay is a common method for assessing protein distribution and directionality in collectively migrating cells (Cory, 2011). This method is advantageous because it does not require chemical attractants in order to induce migration. Instead, the scratch-wound assay procedure typically involves culturing a sheet of cells on top of extracellular matrix. The matrix operates like tracks that allow the cells to migrate. A needle with a fine filamentous tip is then used to etch a wound onto the sheet of tissue. The wound is then left to heal via cellular proliferation and migration, and the direction of migration can be inferred as into the wound.
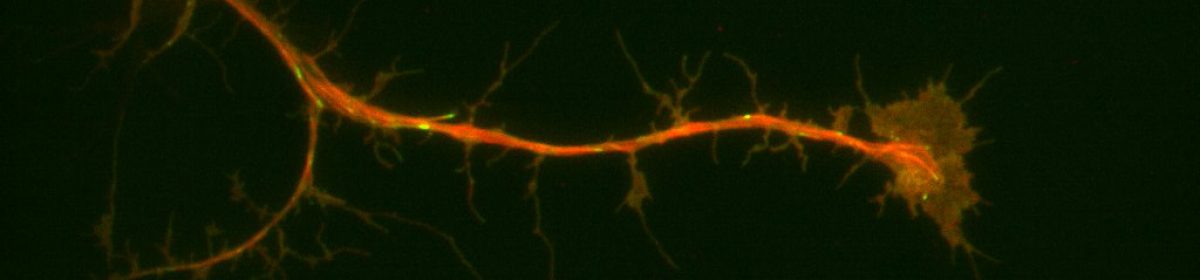
For my thesis, I will be using scratch-wound assays in order to study orientation of microtubule growth in migrating Drosophila cells. I am currently culturing sheets of sheets of RasV12;WTSRNAi cells. Some of the cultures have depleted levels of either the protein Short-stop (Shot) or Pickled eggs (Pigs), both of which crosslink actin and microtubules. Into all of these cultures, I have transfected in a DNA construct that expresses a protein called Eb1, which marks the growing ends of microtubule filaments, that is tagged with a red fluorescent molecule. After performing a scratch-wound assay, I can take three minute timelapses of the migrating cells using Total Internal Reflection Fluorescence (TIRF) microscopy. TIRF microscopy essentially uses a laser to excite the red fluorescent molecule attached to the Eb1 proteins on the growing ends of microtubules. The results are short movies in which we can see red comets marking microtubule growth. One of the goals of my thesis is to calculate the angles at which the microtubules are growing relative to the line of the wound and see if there is a difference in orientation of growth between conditions in which proteins have been depleted in cells.
References:
Cory, Giles. “Scratch-wound assay.” Cell Migration. Humana Press, 2011. 25-30.