I made two points about the 3-D aspects of nucleophilic substitution today in lecture:
- the bimolecular reaction that I described (aka SN2) occurs by “backside attack”. This is an experimental observation that has been made hundreds (probably tens of thousands) of times for a range of compounds. I could have added that molecular models also predict that backside attack is preferred, i.e., transition state models for backside attack are always lower in energy than transition state models for frontside attack;
- the preference for backside attack can be rationalized by looking at how the nucleophile’s HOMO and the substrate’s LUMO overlap. Backside attack allows good overlap and the construction of a new, delocalized orbital in which the electrons originally residing on the nucleophile spread over the electrophilic carbon and the leaving group (see below left). The interactions in this three-atom orbital are bonding between Nu and C and antibonding between C and Lg. So, the nucleophile’s “attack” actually weakens the C-Lg bond.
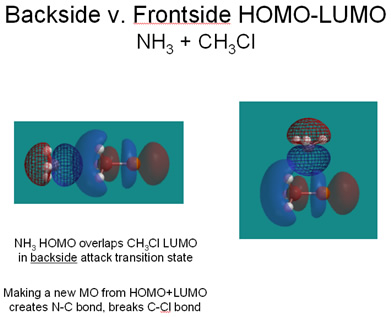
It’s worth pointing out that the diagram in Solomons & Fryhle, p. 227 is labeled incorrectly. First, the phases or numerical signs of the orbital values are not shown, which is not helpful. Second, the “antibonding orbital” and “bonding orbital” labels make no sense; ignore them. On the other hand, you might find it helpful to re-read “The Chemistry Of … HOMOs and LUMOs in Reactions” on p. 97.