Here are pictures of the models that I shared with you in class on Monday. They address different issues.
Model #1 – A Stable Bromonium Ion. A bromonium can be stabilized by placing large groups around the alkene. These groups offer steric hindrance to the bromide anion so that backside attack can’t occur.
Notice that the “alkene” carbons in the reactant lose their planar geometry when bromine bonds to them. This geometry change pushes the bulky substituents downward where they block the path of any nucleophile that approaches from the backside. Since this also increases the exposure of the frontside of these carbon atoms, you might regard the stability of this ion as further evidence that SN2 reactions require backside attack.
Model #2 – Unsymmetrical alkene leads to unsymmetrical bromonium ion, plus SN1-SN2 ring-opening. An unsymmetrical alkene like Me2C=CH2 produces a geometrically distorted bromonium ion like the following:
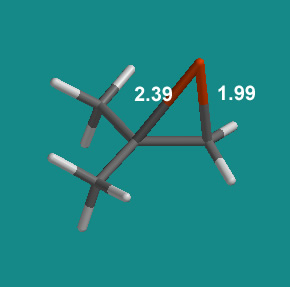 Notice that one CBr bond is much longer than the other, 2.39 v. 1.99 A. A normal CBr single bond is 1.95 A, so one bond in the bromonium ion is almost “normal” while the other is considerably weaker.
Notice that one CBr bond is much longer than the other, 2.39 v. 1.99 A. A normal CBr single bond is 1.95 A, so one bond in the bromonium ion is almost “normal” while the other is considerably weaker.
We explained the distorted geometry by pointing out that 1) it
reduces angle strain around the CH2 carbon, and 2) it is accompanied by
a shift in electron density from the more substituted carbon to bromine
(which is, after all, the more electronegative atom). This leaves the
more substituted carbon with a partial positive charge which is
stabilized by the two methyl groups (hyperconjugation + polarization).
Another
way to look at this structure is to say that bromine is starting to
“leave” the more substituted carbon without waiting for a nucleophile
to replace it, i.e., the geometry is beginning to approach that of an
SN1 transition state. However, to dislodge the bromine completely, the
nucleophile must enter from the backside, which is reminiscent of an SN2 transition state. Hence, we might call this an “SN1-SN2 reaction”.
It
is important to remember that this odd behavior is due to the
unsymmetrical substitution pattern in the alkene. If both carbons are
substituted, the intermediate is less distorted and a mixture of
products might result.
