Professor Hendrik Zipse, Ludwig Maximilians Universität-München, has posted a nice set of computational chemistry teaching resources on his research group’s web site. There are explanations (auf English!) of many basic computational concepts and procedures, along with how-to guides for using mainline modeling programs. Maybe the best way to begin (after you click the link above) is to click “Table of Contents” and go from there.
Learning Computational Chemistry from Prof. Zipse
Predicting NMR shifts for organoiodine compounds in Spartan
Shift prediction for RI (and ArI and I-anything) molecules is impossible using the 6-31G*basis set because these basis functions have not been defined for iodine. Use 6-311G*.
‘Out of Memory’ Errors in Spartan
The computers in C203 are equipped with i7 8-core processors and 8G of total RAM. This occasionally leads to “out of memory” errors. These errors are particularly common during Spartan DFT IR calculations (a typical output error message reads, “Error Occurred: Not enough total memory”).
The error occurs because of a mismatch between the available memory and Spartan’s expectations regarding memory. Spartan normally expects to use all 8 cores and to find 2G/core, but not all Spartan calculations actually demand this much memory. When a Spartan calculation actually demands all of the expected memory, e.g., the situation that occurs in DFT IR calculations of a certain size, Spartan discovers that the memory it expects (16G total) exceeds the memory available (8G or less), and an “out of memory” error is the result.
Spartan tip – Natural Bond Orbitals
Parts of Weinhold’s Natural Population Analysis (NAO, NBO, but not NHO, NRT, perturbation calculations, etc.) have been part of Spartan from the very beginning, but the instructions for accessing this analysis keeps changing. Here is my understanding of what parts you can access in Spartan’14 and how to get them:
- Natural atomic charges – By default, all successful quantum calculations (HF, DFT, etc.) in Spartan’14 are followed automatically by calculations of electrostatic, Mulliken, and Natural charges, i.e., charges based on Weinhold’s Natural Atomic Orbitals (NAO). The easiest way to see these charges is to click Display: Properties and then click the atom of interest.
- Another way to see charges is to click Model: Configure and then select the charge of interest. All of the charges will be displayed on the model simultaneously.
- Yet another way to see charges is to click Setup: Calculations and then click the Print: Charges & Bond Orders. This will add a list of Natural charges to the text output (Display: Output).
- Natural Atomic Orbital (NAO) populations – An atom’s Natural charge can be parsed into NAO populations. Normally, one expects large populations for compact low-energy orbitals (labeled “MIN”) and vanishingly small populations for diffuse high-energy orbitals (labeled “RYD”). A list of these orbitals and their populations can be generated in the text output by entering the keywords NBO PROPPRINTLEV=2 in Setup: Calculations.
- Natural Bond Orbital (NBO) definitions and populations – NBO usually correspond to the bond orbitals one might anticipate by looking at a molecule’s Lewis structure, e.g., the NBO of methane include 4 CH sigma bonding orbitals. A list of these orbitals (broken down into NAO contributions from each atom) and their populations can be generated in the text output by entering the keywords NBO PROPPRINTLEV=2 in Setup: Calculations.
- Natural Bond Orders – A list of bonds and numerical Natural bond orders can be generated in the text output by entering the keywords NBO PROPPRINTLEV=2 in Setup: Calculations.
- Controlling the output – More control over the appearance of the NBO can be achieved by combining PROPPRINTLEV=2 with one of the following keywords: NBO=NORMAL or NBO=IONIC or NBO=3C. However, if one really wants to access the full sophistication of NBO analysis, it probably makes better sense to use the NBO6 program itself.
Predicting bioactivity of toxic chemicals
Here’s the set up: I draw three chemical structures on the board, all of them unfamiliar. Then I ask you some questions: Which one has the strongest odor? Which one raises your body temperature the most? Which one is most effective at strengthening long-term memory? Which one is most effective at curing a case of acne? Which one is most effective at reducing arthritis pain?
All of these questions ask you to make a predictive leap, starting from a chemical structure and ending at a biological phenomenon, what we call ‘bioactivity‘. As you might expect, this ability to make structure-to-bioactivity predictions is critical in the development of new medicines. However, it could also play a vital role in protecting people from hazardous chemicals. To learn more about predicting the bioactivity of toxic chemicals, check out “Crowdsourcing Toxicity Prediction” (C&E News, 1 July 2013, p. 18). This could be a good thesis topic…
Spartan tip – Symmetry errors
Spartan automatically examines models for symmetry. When it finds symmetry elements, it applies these in subsequent calculations. For example, if you build ‘flat’ NH3 (possible point groups: Cs, C3v, D3h, etc.) and calculate its equilibrium geometry, you will end up with a flat ‘minimum’. In other words, your final model won’t be a minimum at all. Here are some tips for dealing with symmetry, wanted and unwanted:
- turning off symmetry constraints
- uncheck the Symmetry box in Setup: Calculations
- adjust an atom (or group) position so that the initial model is less symmetric (if Spartan lists the point group as C1, no symmetry has been detected)
- “<point group> slipped through” error message prevents the desired calculation – either uncheck the Symmetry box (see above) or Minimize a few times to reach a higher symmetry model (this can be a disaster so remember that you can use Undo to reverse one Minimize)
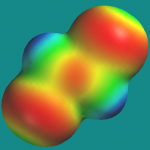
Can a picture tell you whether a molecule is polar?
This question is asked in a particularly interesting way by G.E. Höst et al in the December issue of J. Chem. Ed. (“Students’ Use of Three Different Visual Representations to Interpret Whether Molecules are Polar or Nonpolar”, DOI: 10.1021/ed2001895). They presented students with three types of pictures:
- positive and negative isopotential surfaces rendered in blue and red (RB)
- an electrostatic potential map on an isodensity surface (MAP)
- an isopotential surface for the node in potential, i.e., the surface(s) that separate regions of positive and negative potential (ISO)
and they correlated student performance, i.e., whether a student could correctly say whether a molecule was polar or nonpolar, and how long it took for them to reach that decision, with the type of picture. Here are ISO and MAP pictures of trans-1,2-dichloroethene:
Adjust JPEG resolution in Spartan’08 (Windows)
This info has been knocking around my email inbox for a long time (rec’d from Wavefunction help on 9/15/2009), but while Spartan has moved on to Spartan’10 (’12 or ’13 just around the corner), maybe the advice is still useful.
Sabbatical Winds Down … iSpartan Winds Up
My 2011-12 sabbatical is entering its final weeks and I am already receiving emails about college business that supposedly only I can deal with. Sigh.
So whatever happened to that long list of possible sabbatical projects I outlined last August (Bond, Valence Bond)? Well, a lot actually.
- Wavefunction and I submitted an SBIR grant proposal to NSF last summer to develop software for the iPad. In the Fall we received our rejection letter. Our proposal was: Not good enough. Not original enough. Not enough potential market impact. (To be honest, I have forgotten what their reasons for rejection were. We move on.)
- So, in that "move on" spirit … Wavefunction and I continued work on a molecular modeling app for the iPad. I traveled to the ACS national meeting in Denver, CO, last September and demo'd an early version of the app to everyone who stopped at the Wavefunction booth. Design and development continued through the winter and spring and, just last week, the new iSpartan app made its debut in the iTunes App Store. It's a pretty slick way to do serious molecular modeling. To build a model, you literally sketch molecules the way you would with pencil and paper (the old-fashioned "click" to draw bonds, atoms, and rings is largely gone), press a button, and say, "shazam!" (or maybe "spartan!") The sketch instantly turns into a 3D model. With a few more taps you can animate vibrations, display orbitals, and see calculated NMR spectra. To get full details on iSpartan, visit Wavefunction's iSpartan info page.
- The Denver ACS meeting was useful for another reason as well. My Ph.D. adviser, Prof. Charles Casey, stopped by the Wavefunction booth to say hello and we got to talking about some of the research projects that he was working on. One of them involved predicting proton NMR chemical shifts for an isotopically labeled molecule that an old friend and fellow grad student, Dr. Mark Cesa, had made as part of his thesis research. By March we had calculated the data we needed to settle a long-running research problem and by June we had submitted an article to Organometallics for publication. The referees recently gave us "two thumbs up" (along with two lists of revisions) and the final draft will be back in the editor's hands before the end of the month.
- And, finally, all this month (and all next month and well into next semester) I am keeping busy writing POGIL learning activities to be used in Chem 201 this fall. It is a painfully slow process (3-5 days of writing per activity; Andrei Straumanis – I can't thank you enough! This would have been impossible without you.), but I am really excited about the product. I think the students and I are going to have a blast working on these in class.
And what will I do next summer? Take another look at those book ideas? Or work on a new version of iSpartan or perhaps Odyssey for the iPad? Or, maybe even a closer look at valence bond models for organic chemistry? Who knows?
Converting Total Energy to Enthalpy, 298K, 1 atm
The total energy is obtained from a model in which the nuclei are frozen in place. Therefore, the total energy fails to include the kinetic energy of the nuclei and cannot be compared directly to the standard enthalpy, H, at 298 K, 1 atm. This post describes the procedure for obtaining the missing kinetic energy (and the standard gas-phase enthalpy) with SPARTAN'10 using the EDF2/6-31G* method.
Calculations for methanol, CH3OH, are used to illustrate these method. To follow this example on your own, build methanol and calculate its Equilibrium Geometry at Ground State with Density Functional EDF2 6-31G* in Vacuum. Set Total Charge: Neutral and Multiplicity: Singlet and check the boxes next to IR and Thermodynamics.