Yesterday’s discussion of Fe-TAML vs. Fe-porphyrin gave me an opportunity to spout off on some subjects that I know very little about. In an effort to provide some more reliable information, I’m going to post some info on Fe-porphyrin complexes. Here’s the first installment.
(Note: most of the following links are to Wikipedia pages. I make no promises about the accuracy or usefulness of the info provided. It was simply the most convenient way for me to make links. The image of HRP was obtained by “searching” Google images and taking the first likely-looking picture.)
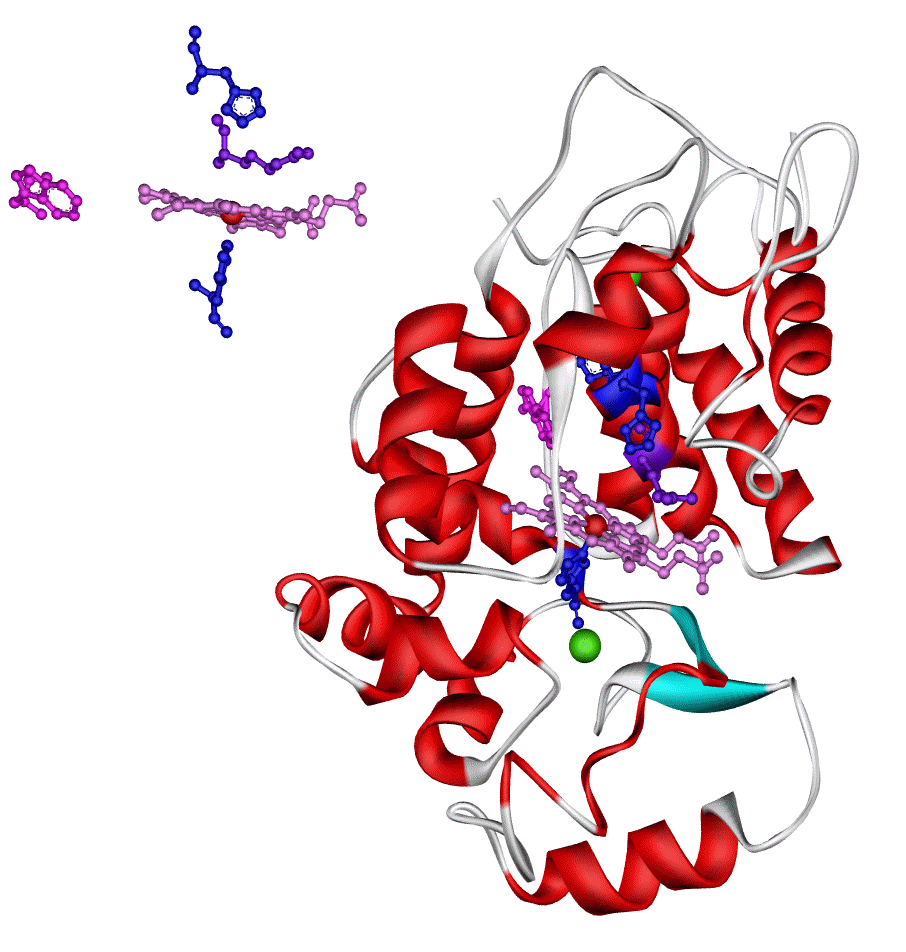
The following image shows a crystal structure of horseradish peroxidase, HRP, as determined by x-ray diffraction. The image was taken from the web page of Dr. Shyamalava Mazumdar and you can find more information about this enzyme, and others, on that page.
Some features of interest (fyi – click on image to enlarge it):
- There are actually two images here. The image in the upper left shows Fe (red sphere) surrounded by the porphyrin ligand (violet), and some axial ligands (blue). I can’t explain all of the groups that appear in this image, but if you can identify the Fe-porphyrin unit here, you can use these colors and shapes to locate this unit in the peroxidase enzyme.
- The larger image, lower right, shows the protein (ribbon format), the Fe-porphyrin cofactor embedded in it, and some other atoms as well.
- The “curly pasta” (red) sections of the protein are referred to as “alpha helices“. This structure permits a large number of intrahelix hydrogen bonds and is a common feature in proteins.
- The gray sections of the protein are sometimes called “random coils” because they don’t follow any structural pattern.
Note: feel free to add comments. For example, links to better information about these topics, or links to other Fe-porphyrin protein images, would be welcome. Questions are also welcome, but you can always ask those in person when I finally get to work.