Of Flies and Minds
Schizophrenia is a neurodevelopmental disorder and it is caused when the brain is not formed normally in early life. Some of the symptoms can include delusions, hallucinations, disorganized speech, trouble with thinking, and lack of motivation. Emerging studies are showing that Schizophrenia can impede neuronal network formation and neurodevelopmental processes such as differentiation and migration(Berreta, 2016). Since The Extracellular Matrix (ECM) plays a key role in the regulation of cell differentiation and migration and axonal outgrowth and guidance (Bandtlow and Zimmermann, 2000), we predict that aberrant interaction between the neurons and(ECM) could cause disruption observed in Schizophrenia.
Our project is studying the neuronal-ECM relationships, and how ECM signaling dictates the differentiation of neuroblasts, axonal extensions, and neuronal architecture of neurons and is comparing these developments in terms of their axonal extension across different substrates of ECM and Concanavalin A (Con A). Our long-term goal is to understand what could cause the aberrant interaction between neurons and ECM.
ECM is a large network of proteins and other molecules that surround, support, and give structure to cells and tissues in the body. It plays a critical role in dictating cellular behavior. In addition, ECM also helps cells attach to, and communicate with nearby cells and plays an important role in cell growth, movement, and other cell functions. Con- A, our other substrate, is a lectin that does not engage ECM receptors and thus does not convey vital signaling information to neurons. Con A is used because the cells stick well but there is no real biologically significant signaling that is happening. Hence using it as a substrate could act as a control to aid our understanding of how ECM dictates neuronal behavior and how neurons across species interact with their extracellular environment.
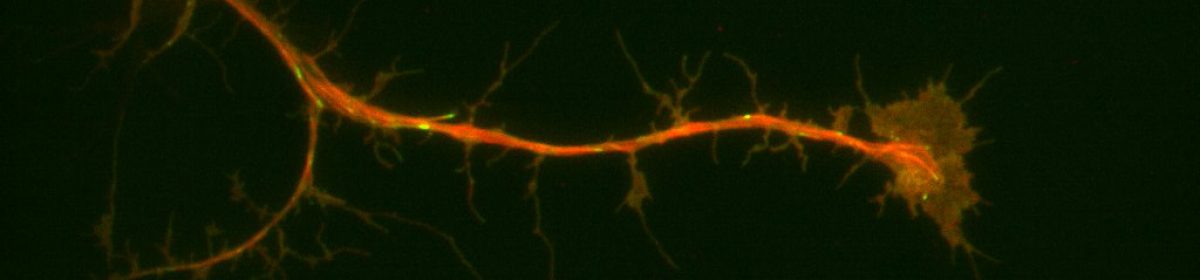
Neuronal progenitor cells from the brain of Drosophila melanogaster (fruit flies) is our preferred system to study the neuronal-ECM relationships. During neurogenesis, neuroblasts undergo a series of divisions either producing more of themselves or differentiating into neurons or glia1. Brains from larvae, which are enriched in neuroblasts, were removed and cultured for days. This system is ideal for detailed, high-resolution microscopy and exploring signaling from the ECM since it has formerly led to fundamental discoveries furthering our understanding of neurons and neurogenesis. Additionally, purified ECM is more biologically relevant because when coupled with compliant substrates, it mimics the softer environments of brains representing a powerful system allowing us to probe ECM-neuronal interactions from several different axes. Our preliminary data suggested that neurons plated on nECM behave differently than those plated on Con A. We collected Neuroblasts from third-instar larval with the genetic background of (Prospero-GAL4xUAS-EB1::EGFP; UAS-Jupiter::mCherry) and plated them on the substrate of either ECM or ConA. We used TIRF microscopy for imaging and observing the development of our neurons. In parallel with our predictions, we replicated former lab findings demonstrating that microtubule dynamics and axonal extensions increased in neurons plated on ECM compared to con A.
Another line of inquiry we pursued was using the UAS-RNAi fly line in combination with the inscrutable-Gal4 driver to deplete the major adhesion molecule, Syndecan, and observe neuroblast behavior when plated on ECM and Con A. Interestingly, the results we got from these were quite surprising. The neurons plated on ECM did not show much growth and had axonal extensions. The neurons were unable to sense the signaling from the ECM and did not develop or show axonal extensions; instead, they either died or showed minimal development. This result suggests that Syndecan might contribute significantly to the regulation of the neuronal-ECM interaction. Furthermore, it could have an impact on the axonal growth of neurons on ECM, neuronal differentiation, and overall neuronal development which in turn might contribute to the cause of SZ. We are currently working on replicating these results and I am excited to see what we will find out.
Berretta, S. (2012a, March). Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3234338/#R18
Berretta, S. (2012b, March). Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3234338/#R18
Stephen J Glatt, Stephen V Faraone, Ming T Tsuang. (n.d.). Is Schizophrenia A Neurodevelopmental Disorder?. Academic.oup.com. https://academic.oup.com/book/40600/chapter-abstract/348206860?redirectedFrom=fulltex