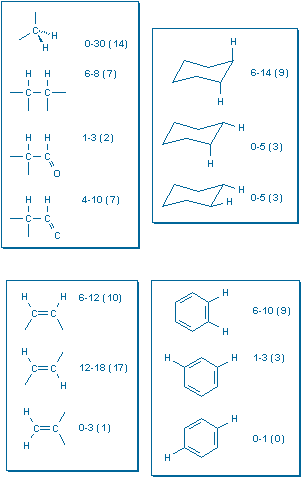
NMR & IR Tables
1H NMR H-H Coupling Constants
Typical coupling constants (in Hz) are listed in the following chart (the chart has been adapted from a reserve text, Sorrell, Interpreting Spectra of Organic Molecules, University Science Books, 1988, p. 69). The usual range in J value, and the “most frequently observed” J value (in parentheses), are both listed.