Grignard Synthesis of an Aromatic Acid
Procedure
Computer Lab (Week 1)
Before starting wet lab work, you will first predict the final products for each of the six aryl bromides. Next, you will search for physical properties and spectral data for the six aryl carboxylic acids. You will use the physical properties and spectral data you find to devise a plan to identify your aryl carboxylic acid and starting aryl bromide.
Pre-lab
The starting aryl bromides are stored in the reagent hoods in bottles marked with a color code. The solvent that you need to run the reaction is also located in this fume hood.
Because you won’t know which aryl bromide you are going to use, prepare a Table of Physical Constants, and a table of hazard/disposal information for the following compounds:
- 6 aryl bromides (bromobenzene (X = H), para-bromoanisole (X = 4-OCH3), meta-bromoanisole (X = 3-OCH3), 4-bromonitrobenzene (X = 4-NO2), 4–bromotoluene (X = 4-CH3), and 2-bromotoluene (X = 2-CH3))
- 6 corresponding aryl carboxylic acids
- diethyl ether
- hexanes
- toluene
- magnesium metal
- CO2 “dry ice” (Frostbite hazard – handle with THICK gloves, no disposal required)
- 10% aq. HCl
- 5% aq. NaOH
- CaSO4 or Drierite (spent reagent looks pink or purple; place in labeled waste jar)
You do not have to look up the physical properties of the Grignard reagents because you will not be isolating them, but you should still acquaint yourself (and make notes in your notebook) about their chemical reactivity and handling. For your information, all Grignard reagents are strong bases that react rapidly with water (corrosive, avoid skin and eye contact). They can be disposed of (when necessary) by carefully combining them with a weak acid, like water. This gives an organic compound that can be disposed of in the usual way.
Two reagents, diethyl ether and CO2, will be combined with the Grignard reagents. These reagents must be kept as water-free as possible. Both reagents absorb water from the air (this is especially true of “dry” ice). Therefore, use the special anhydrous ether in the reagent hood and keep this bottle tightly capped when not in use. Likewise, do not obtain dry ice until you are ready to use it, and use FRESH dry ice.
Reaction (Week 2)
Conduct all operations in a fume hood.
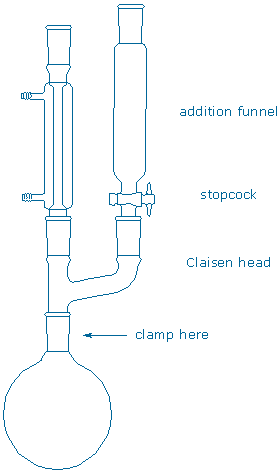
Assemble a 100 mL round bottom flask, magnetic stir bar, Claisen head, addition funnel, and reflux condenser [NOTE]The apparatus without the two drying tubes is shown below. The drying tubes are inserted in the reflux condenser and addition funnel after the apparatus is dried with a heat gun.. Prepare two drying tube with fresh drying agent , but do not attach them to the apparatus yet. (The drying agent contains white crystals and colored crystals. The latter should be blue. Purple crystals indicate a water-saturated drying agent and these should be discarded in the appropriate container).
Remove the hoses from your condenser, and remove the stopcock and cap from your addition funnel. Carefully dry the apparatus using a heat gun (FIRE/BURN hazard [NOTE]The heat gun gets extremely hot. Always hold it by its handle (never grab the nozzle). Remove all solvents and flammable reagents from the hood before using the gun (your apparatus should not contain any compounds at this point).). Start heating at the bottom and work your way towards the top. After you finish and the apparatus is still somewhat warm to the touch, place the drying tubes on top of the reflux condenser and addition funnel, and insert the stopcock in the addition funnel.
After the apparatus is cool to the touch, charge the r.b.f. with magnesium metal (310 mg) and anhydrous ether (8 mL) and then some iodine flakes. Place a solution of the aryl bromide (1.25 mL) and anhydrous ether (8 mL) in the addition funnel and replace the drying tube on top of the funnel. Add approximately 1/3 of the aryl bromide solution to the flask while stirring. Place a ceramic heater under the flask and reflux the solution. As soon as the orange color of the iodine begins to fade, and the Grignard reaction begins, add the rest of the aryl bromide solution to the flask [NOTE]If no reaction occurs between magnesium and the aryl bromide, you will see shiny magnesium metal resting at the bottom of a clear, colorless solution. If a reaction does occur, the metal will become dull and the solution will turn cloudy. The reaction is exothermic, so if it starts on its own, the ether will begin to boil spontaneously. Grignard reactions can be notoriously difficult to start. This is partly due to a thin metal oxide coating on the surface of the magnesium metal. Stirring and heating the mixture often breaks down the oxide coating and initiates the reaction. Other recipes for starting reluctant reactions will be discussed in lab.. After all of the aryl bromide solution has been added, gently reflux the mixture for another 30 minutes, then let it cool.
When the reaction mixture is cool, obtain freshly crushed dry ice (3 g) and place it in a large dry beaker. Pour the reaction mixture slowly over the entire surface of the dry ice, then stir the mixture with a dry spatula or glass rod until no dry ice can be observed.
Workup (Week 2)
Add 10% aqueous HCl (7 mL) to the mixture and stir. Add wash-grade ether (30 mL) to the mixture and continue stirring until little or no solids are visible. Transfer the mixture to a separatory funnel [NOTE]Use a separatory funnel that is large enough so that it will never more than half full.. Discard the aqueous layer and wash the organic layer once with water (10 mL). Extract the organic layer with 5% aqueous NaOH (2 x 15 mL) and combine the basic extracts in a beaker.
Gently heat the basic extracts to remove any dissolved ether. Precipitate the aromatic acid with a minimum amount of 10% aqueous HCl [NOTE]The products are somewhat water-soluble. Therefore, it is important to use a minimum amount of 10% HCl. Measure the pH as you add HCl and stop when the pH is ~1.. Cool the mixture for 10-15 minutes with an ice bath, then collect the precipitate by vacuum filtration. Transfer the precipitate to a watch glass. If you have time proceed to the crystallization, if not then let it air-dry for at least one week in a designated cabinet.
Purify your crude carboxylic acid product using a mixed solvents crystallization (toluene/hexanes). The products dissolve readily in hot toluene, but not in hexanes. Begin by adding boiling toluene to your product drop-wise to dissolve it. If after the addition of toluene drop-wise, the majority of your product dissolves, but you still have small particles that do not then perform a hot filtration to remove these impurities then continue with the recrystallization procedure. If there is too much toluene following the hot filtration step then you may need to reduce the volume of toluene before you can continue with the recrystallization procedure.
Next, carefully add boiling hexanes until solids reappear or the solution becomes cloudy. Then add boiling toluene again until the solution becomes clear. Very little additional toluene should be required during the second addition. Set your solution aside to crystallize. Collect your final product by vacuum filtration using a Hirsch funnel and let it air-dry before collecting characterization data.
Melting Point Analysis (Week 3)
Weigh your dry recrystallized product and measure its melting temperature range. Based on this range, establish two plausible identities from the list of possible aryl carboxylic acids in your physical properties table.
Once you have determined two possible acid identities, prepare two mixtures: your product+authentic acid 1 and your product+authentic acid 2. Mix equal amounts of each compound using roughly the amounts that you would typically insert in a melting point capillary tube. Transfer the mixture to a capillary tube, then measure the melting temperature as a range from the onset point until the sample is fully melted.
Spectroscopic Analysis (Week 3)
Confirm the identity of your carboxylic acid by collecting spectroscopic data.
