Grignard Synthesis of an Aromatic Acid
Background
Organometallic Reagents
An organometallic reagent is a compound that contains a carbon-metal bond. The two most frequently used organometallic reagents are organolithium reagents, RLi, and Grignard reagents, RMgX. Both reagents have been and continue to be the most important reagents for forming carbon-carbon bonds. The popular Grignard, Aldol, and Wittig reactions all make use of organometallic reagents.
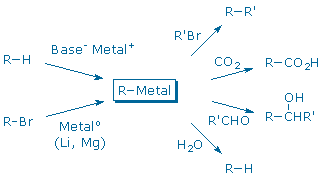
The most common routes for forming and using organometallic reagents are shown below.
Some organometallic reagents can be prepared by deprotonation of an appropriate carbon acid (R-H) with a strong base (Base– Metal+). Another useful method involves the reduction of an organic halide, R-Br, with a neutral metal, like Li or Mg (Victor Grignard, the discoverer of the latter reaction, was awarded the 1912 Nobel Prize in Chemistry for this discovery).
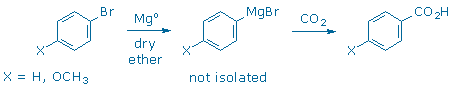
Once it is formed, the organometallic reagent can react with a range of carbon electrophiles to form new carbon-carbon bonds. This experiment illustrates the synthesis of a Grignard reagent, ArMgBr, from an aryl bromide, ArBr. The Grignard reagent is not isolated. Instead, it is immediately combined with CO2 (dry ice) to make an aromatic carboxylic acid, ArCO2H. Another experiment in this manual, Synthesis of Limonene, illustrates the deprotonation route used in the Wittig reaction.
Although organometallic reagents are useful, they are also highly reactive bases that react rapidly and irreversibly with water. Particular care must be taken to prevent contact between water and an organometallic reagent. You must carefully dry all glassware that will come into contact with the reagent, and remove traces of water from solvents and other reagents that will be mixed with the reagent. This experiment will use anhydrous diethyl ether, a commercially available solvent.
Synthesis of an Unknown Aromatic Acid
Six alternative starting materials will be provided for this reaction: bromobenzene (X = H), para-bromoanisole (X = 4-OCH3), meta-bromoanisole (X = 3-OCH3), 4-bromonitrobenzene (X = 4-NO2), 4-bromotoluene (X = 4-CH3), and 2-bromotoluene (X = 2-CH3). Groups of three students will be assigned three different aryl bromides. Each student will attempt to make the corresponding carboxylic acid. For example, bromobenzene should yield benzoic acid, while para-bromoanisole should yield para-anisic acid, etc.
The resulting carboxylic acids are solids so this experiment also provides a chance to revisit some of the experimental challenges of purifying and characterizing solids. Assuming you eventually obtain a solid product, you will attempt to purify it using a two-solvent recrystallization technique. Then you will identify your product using spectroscopic methods of your choice (IR, NMR, GC). In addition you will measure the melting temperature of your recrystallized product and compare it to the published melting point. Finally, you will combine your product with an authentic carboxylic acid sample, and measure “mixed melting points”. Mixing your product with more of the same should not affect its melting point. Mixing your product with a different carboxylic acid, however, should give a mixture that begins melting at a lower temperature and melts over a broader temperature range.