Distillation of a Liquid Mixture
Procedure
For this experiment you will work in two-person teams as follows. Choose a partner and write their name in your lab notebook. Obtain about 44 mL of a whiskey sample and split it 50:50 with your partner so that each of you has about 22 mL of sample. Write the name of the whiskey sample in your lab notebook.
During week 1, you and your partner will perform independent simple distillations of your respective 22 mL samples. Then you will recombine your samples and, working together, perform a fractional distillation on the 44 mL sample. Each of you will keep one compound from the fractional distillation. During week 2, you will measure the FT-IR spectrum of the compound you kept and determine its odor. When the lab is complete, you will exchange T vs. V data for your simple distillations, as well as FT-IR spectra, so that each of you has a complete set of data on all distillations and both compounds. Each of you will be responsible for writing up your own lab report.
Pre-lab preparation
There is a particular routine we would like you to follow in preparing for lab work each week. Please read preparing for lab in the Policies section of the manual so that you know what is expected.
A key step in these preparations is the preparation of your lab notebook before you come to lab. Because this is the first time you will be using your notebook, you will need to prepare the outside and inside covers, establish a table of contents, and begin numbering pages. Note: these things only need to be done once this semester.
Next, you will need to enter the information that is expected on the right and left-hand pages. Much of this information, e.g., the title, the chemical equation/materials, the table of physical properties, will need to be entered just once at the beginning of each new experiment. If an experiment runs multiple weeks, you won’t need to refresh this information.
However, some information will need to be added every week. This information is your work plan for the week (a complete plan should also include reminders about safety hazards and disposal). Because a plan is not part of the official record, enter your work plan on the left-hand pages. We expect you to follow the plan that you have written in your notebook. You should not need to refer to the lab manual during lab. A blank left-hand page means you don’t have any work plans for the day so please make sure you attend to this before you come to lab. (Students who arrive unprepared can expect to be sent out of the lab to prepare.)
We will set-up the lab notebook and prepare the first pre-lab during lab. You will have a chance to ask questions and you will also be able to check your work before submitting scans of your pre-lab for Experiment 1.
Background
Normally, the background information for an experiment will be presented on the experiment’s Background page. We didn’t follow this pattern here because we thought that the theoretical description of distillation already made for lengthy reading. The following is a brief introduction to what you are actually going to do in this experiment and why. This introduction will be followed by experimental procedures for weeks 1 and 2 of this experiment.
This experiment is based on a recently published article, “Using Whiskey Flavoring Compounds to Teach Distillation and IR Spectroscopy to First Semester Organic Chemistry Students.” by H. Wan et al. (J. Chem. Educ. 2014, 91, 123-125, DOI: 10.1021/ed300718y). The ‘story’ that drives this experiment is that you (and a lab partner) have been sent a whiskey sample from an (imaginary) whiskey distillery and your tasks are 1) to determine how many compounds are responsible for each whiskey’s unique flavor, and 2) to identify the structures of these flavoring compounds. As you do this, you will also make measurements that will allow you to compare the effectiveness of simple and fractional distillation techniques at separating your mixture. (And we hope that these data and experiences will provide a useful mental framework for performing successful distillations in the future.)
As for the central task, determining the chemical composition of a mixture, this kind of task rears its head on a daily basis in the typical organic chemistry lab. That makes this experiment a good introduction to the work that chemists do. The experiment also features two important laboratory techniques, distillation and IR spectroscopy, that will be used several more times during the year.
To make the separation and identification more manageable, three simplifications have been introduced:
- First, the major ingredients from each “whiskey,” namely, water and ethanol, have already been removed. Your sample contains only the flavoring compounds.
- Second, you can expect each sample to contain only two compounds and the sample to contain equal volumes of each compound (real whiskeys contain many flavoring compounds in varying amounts). Note that this is only an expectation. It is still your task to collect and analyze data that will confirm or deny this proposition.
- Third, both compounds can be found on this list (organized by functional group):
- Alcohols: 2-propanol, 1-pentanol, 3-methyl-1-butanol
- Ketones: 2-butanone, 2-pentanone
- Esters: ethyl acetate, ethyl butyrate
Notebook preparation
In preparing your lab notebook for this experiment, do not write a chemical equation underneath the title because you are not going to attempt a chemical reaction. Instead, under the title, write “Materials” and draw a structural formula for each of the 7 possible compounds, and then write their names next to their formulas.
Follow the Materials with a Table of Physical Properties that lists all 7 compounds, their molecular weights (MW), melting points (mp), boiling points (bp), and densities (g/mL) (specific gravity relative to water can also be used). These data can be obtained either from the CRC Handbook, ChemSpider, or Sigma/Aldrich chemical catalog (see Lab Links on the manual’s sidebar). For an example of a completed Table of Physical Properties, see Sample Notebook Entry in (How to Keep a Laboratory Notebook).
Next, prepare a safety hazards and disposal section based on the known properties of these 7 compounds. Safety information can be found by looking up the SDS for each compound (see Lab Links on the manual’s sidebar). Three standard questions to ask yourself are:
- Is this compound safe to heat? After all, you plan to distill it.
- Is this compound reactive towards other compounds, or air, or water? You plan to heat this compound as a mixture with one of the 6 other compounds. Will they react when heated? Also, air and water (vapor and liquid) are widespread in our lab so unintentional contact with these substances is unavoidable.
- Does this compound pose any health hazards, either short- or long-term? If so, what kinds of exposure (vapor, direct contact with liquid, ingestion) should you be concerned about?
You may not know the answers to these questions, but keep a sharp eye out and don’t be afraid to consult with other, more experienced chemists. Should you discover any hazards, (in this case, all 7 compounds are volatile and flammable, and there may be additional hazards as well), list any precautions you should take, e.g., “keep in fume hood, do not work with near open flames.”
Unless you come across warnings in the SDS that indicate special disposal is warranted, all organic compounds can be disposed of in the organic waste container. If this instruction applies to all 7 compounds, you can just write, “Dispose of all organics in the organic waste containe
Week 1: Distillations
Pre-lab videos. Please review the Moodle videos or the following videos on lab procedures before lab (these are all from The Interactive Lab Primer):
- Simple Distillation (full video)
- Fractional Distillation (focus on connection between temperature & liquid collection)
Wear goggles at all times. Carry out all operations in a fume hood.
Distillation equipment from each student’s lab desk
- 100 mL 14/20 round bottom flask (boiling flask)
- Claisen head with side tube
- thermometer and 14/20 adapter
- reflux condenser & water hoses & hose clamps
- vacuum adapter
- 10 mL graduated cylinder (to collect distillate)
- fractionating column w/ beads (fractional distillation only)
- 20 test tubes + test tube rack (to collect distillate from fractional distillation only)
- Three 25 mL Erlenmeyer flasks (to store fractions from fractional distillation only)
Distillation equipment from hood/lab
- boiling chips (in lab)
- lab jack
- ceramic heater + controller
- screw clamps (1 or 2), plastic joint clamps (2)
- water hoses
All glassware must be clean and DRY {tooltip}[NOTE]{end-texte}If your glassware looks clean and dry, use it as is; do not clean it without consulting an instructor first because it will take a long time to dry. Likewise, your fractionating column should be clean and dry already, but show it to an instructor if you think it needs cleaning. Do not attempt to clean it with soap and water since it will take forever to dry.{end-tooltip}.
Each test tube is supposed to hold 2 mL of liquid. Mark this level on each tube with a felt pen or tape {tooltip}[NOTE]{end-texte}Pour 2 mL of acetone into a graduated cylinder then transfer this liquid to a clean dry test tube. Use this test tube to mark the volume level on your other test tubes either by holding it side by side and marking. Or you can pour the acetone into a new test tube, mark it, then transfer it to the next test tube, and repeat until they are all marked. {end-tooltip}.
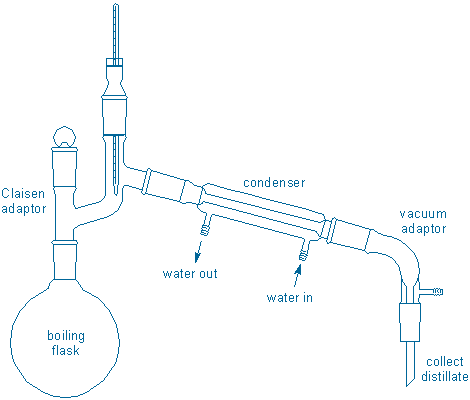
Assemble a simple distillation apparatus in your fume hood:
Assembly tips: You must clamp the boiling flask to the monkey bars and place 3-4 boiling chips in the boiling flask. Do all of this first. {tooltip}[NOTE]{end-texte}Position the boiling flask high enough so that you can place a lab jack + ceramic heater underneath the flask and still be able to raise/lower the lab jack. Also, position the flask so that the water hoses will be able to reach the condenser.{end-tooltip} Add your sample to the flask using a short-stem funnel. Then add the remaining glassware. Adjust the height of the thermometer so that the bulb is just below the opening leading to the condenser, but do not let the bulb touch the glass walls of the apparatus. Water hoses should be attached to the condenser with hose clamps (a slow flow of water is all that is necessary). The condenser should be attached to adjoining glassware with plastic joint clamps (do not use these clamps anywhere else). Collect the distillate in a 10 mL graduated cylinder; because you have more than 10 mL of liquid to distill, have another clean container handy so that you can transfer liquid from the cylinder to this container.
When everything has been assembled, check all ground glass joints to see if they fit together properly (if they don’t fit, vapors will leak from the apparatus leading to loss of material and a potential fire hazard if the vapors come into contact with a hot surface). Check that water is flowing; slow flow is good. Are there 3-4 boiling chips in the flask? Is the ceramic heater plugged into the controller and the controller into the wall outlet? (Never plug the heater directly into a wall outlet.)
After you have checked your setup, begin heating the mixture to a gentle boil and adjust the heater setting until the distillate collects at a regular rate of approximately one drop per second. Record the temperature for every ~1 mL of distillate that comes over. The heater setting may have to be gradually increased to keep the distillation rate more or less uniform. When approximately 9 mL of distillate has been collected, briefly lower the heater so that distillation slows and quickly transfer the liquid from the graduated cylinder to the storage container you have prepared, then raise the heater and continue the distillation. When just 1-2 mL of liquid remains in the boiling flask, stop the distillation by lowering and turning off the heater. Always stop a distillation before the boiling flask becomes dry. Save all of your distillate for fractional distillation.
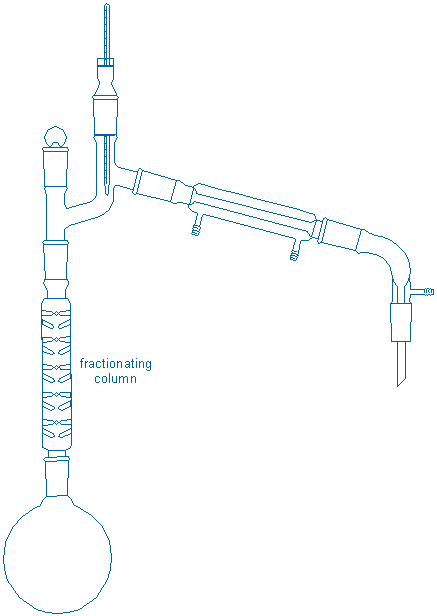
Together with your partner assemble a fractional distillation apparatus in a fume hood:
Assembly tips: All of the tips for simple distillation apply here with the following additions. First, allow the boiling flask to cool back to room temperature before placing the liquid back in the flask. Put both your sample and your partner’s sample in the same boiling flask along with 2-3 fresh boiling chips in the boiling flask. {tooltip}[NOTE]{end-texte}This apparatus is much taller than the previous one. If necessary, readjust the position of the boiling flask so that there is adequate room for a lab jack + heater underneath your boiling flask and also for the thermometer at the top of the apparatus (you may need to readjust the extension of the screw clamps, i.e., pull them away from the bars, to get the height clearance you need).{end-tooltip} Fasten one screw clamp tightly to the boiling flask and loosely fasten a second clamp to either the upper part of the fractionating column or the Claisen head. The first clamp bears the weight of the apparatus while the second clamp provides prevents tipping. After you assemble your apparatus, repeat all of the equipment checks from the previous procedure.
A slow rate of distillation is required to obtain the best results from a fractional distillation. Gradually turn up the heater until the mixture gently boils and adjust the heater setting so that the ring of condensate (vapors) rises slowly up the fractionating column. The rise should be very gradual; this allows the column to achieve a uniform temperature gradient along the length of the column. Apply more heat only when you are sure that the ring of condensate has stopped rising, then increase the heat gradually.
Once you begin to collect distillate, adjust the heater so that roughly 20 drops (~ 1 mL) distills per minute (3 sec/drop) {tooltip}[NOTE]{end-texte} This is a very rough target. Distilling too quickly prevents adequate mixing of gases & liquids in the fractionating column. Distilling too slowly wastes time.{end-tooltip}. Record the temperature when the first drop of liquid in each test tube is collected, and again when the entire 2 mL fraction is collected (collect no more than 2 mL per test tube). Try to keep the distillation rate constant by monitoring the rate and cautiously increasing the heater setting as needed. Stop the distillation when approximately 2 mL are left in the distillation flask or when 20 fractions have been collected. After all the liquid has drained from the column, measure the volume of undistilled liquid.
Troubleshooting your distillation. Most fractional distillations suffer from two problems: flooding and poor insulation.
- Flooding occurs when the rate of distillation is so high that liquid condenses in the column faster than it can drain. Liquid pools in the column (the “flood”) and then begins to boil from this pool. This is unsafe and also leads to poor separation. When flooding occurs, turn off the heater and lower the lab jack. Allow all liquid to drain back into the flask (you may also want to return fractions that were collected during the flooded period back to the flask). Then resume the distillation with a lower heater setting.
- Poor insulation refers to the problem of heat losses from the apparatus that make it hard to sustain distillation. Heat reaches the upper part of the apparatus when hot gas molecules enter the Claisen head and condense. Once the head is hot, vapors can travel to the condenser. Unfortunately, the heating effect of vapor inside the apparatus is offset by heat loss due to air flow over the outside of the apparatus. If you notice that the condensation ring stays in the lower part of the Claisen head and does not rise, this is a sign that heat loss has triumphed. One possible solution is to increase the heater setting so that hot gas molecules enter the upper part of the apparatus at a greater rate, but this “solution” often leads to flooding. Another solution is to reduce heat loss by insulating the Claisen head. You can do this by wrapping the entire head in glass wool or with a single piece of aluminum foil. {tooltip}[NOTE]{end-texte}If you use foil, pinch the foil tightly around each end of the Claisen head so that air flow over the head is reduced, but leave a gap between the foil and the head itself so that hot air can get trapped inside this gap.{end-tooltip}
Once you have collected your fractions and turned off the heater, use your recorded boiling temperatures to identify the two fractions that most likely contain pure samples of the low-boiling compound, and the two fractions that most likely contain pure samples of the high-boiling compound. Transfer these fractions to a clean round-bottom flask, stopper the flask, and wrap the top of the flask with an appropriate sealing material (useful materials will be discussed in lab; parafilm is NOT a useful sealing material for organics) or secure with a keck clip. Stand the labeled flask (Name, Section, Contents) in a beaker and store the beaker under your hood for next week.
Discard all of your remaining fractions and the liquid in your boiling flask. Disassemble your distillation apparatus (clean the apparatus by rinsing it with a small amount of acetone into the organic waste; do not use soap and water unless absolutely necessary) and return the apparatus to your lab desk. Return lab jacks, heaters, hoses, and so on to wherever you found them. Wipe the surface of your hood and lab desk with a damp sponge.
Week 2: Characterization (IR, odor, modeling)
Last week you and your partner distilled your whiskey flavoring and stored “pure” distillates with well-defined boiling temperatures. This week you will characterize these samples in two more ways:
- measuring the distillate’s infra-red (FT-IR) spectrum
- comparing the distillate’s odor to the odors of the 7 compounds that appear in the various whiskey flavorings
Instructions for operating our IR spectrophotometers will be given out in lab. They can also be found here (there is also a link underneath the Appendices menu: Appendices: Instrumentation: FT-IR).
In general, you should never try to identify an unknown compound from its odor. This is very risky. You might damage sensitive cells in your nose. You might even kill yourself if the compound is toxic. Fortunately, the compounds used in this experiment are not that dangerous, but you should still err on the side of caution and reduce your exposure to these vapors as much as possible. Use the technique of “wafting” to experience the odor of your distillate and each of the 7 compounds. What kinds of odors do these compounds have? Is there any correlation between functional group and odor? Or, between molecular size (or shape) and odor?
After you have completed the characterization, you will complete the modeling activity provided in the Moodle. If you run out of time, this activity can be finished outside of lab time.
Lastly, please submit scans of your notebook pages for Experiment 1 to Moodle as a pdf file within 48 hours of the end of your lab. If possible, it is best to upload them at the end of lab so you don’t forget to submit them!
Continue to Report…