Synthesis of Limonene
Procedure
Pre-lab
Prepare a Table of Physical Constants, and a table of hazard/disposal information for the following compounds:
- Week 1 – methyl vinyl ketone, isoprene, 4-acetyl-1-methylcyclohexene [CAS 6090-09-1] , AlCl3 (aluminum trichloride), dichloromethane, diethyl ether, 10% aqueous Na2SO4
- Week 2 – 4-acetyl-1-methylcyclohexene, “instant ylide” reagent (methyltriphenylphosphonium bromide + sodium amide), limonene, THF (tetrahydrofuran), hexane, 10% aqueous K2CO3
As is often the case in chemical research, hazard and disposal information may not be available for all of your compounds. If you cannot find the necessary information, you should still make a “best guess”entry in your notebook for each compound.
AlCl3 is a strong Lewis acid. It reacts vigorously with a variety of Lewis bases, including water (HCl is released when AlCl3 comes into contact with water). Therefore, it must be protected from water,wet solvents, and wet glassware. AlCl3 must be treated chemically before it can be disposed of. Please refer to a MSDS for additional info.
The “instant ylide” reagent contains a strong base, NaNH2. This compound reacts vigorously with water and with other weak acids, releasing NH3. Therefore, “instant ylide” must be protected from water, wet solvents, and wet glassware. “Instant ylide” must also be treated chemically before it can be disposed of. Please refer to a MSDS for additional info (hint: if you can’t find anything information on “instant ylide”, try looking up its components).
Week 1: Diels-Alder Reaction & Workup
Conduct all operations in a fume hood.
Make a solution of MVK (0.15 mL) and isoprene (0.15 mL) in dichloromethane (3-4 mL). Use a dry container of appropriate size. Remember, if the container is too small, some of the reaction mixture might splash or foam over the top (rule-of-thumb: never fill a container more than halfway). If the container is too large, stirring will disperse the mixture over the container’s surface and make it hard to recover your product. Cool the solution in an ice bath, and add a small spatula tip of AlCl3 {tooltip}[NOTE]{end-texte}Since AlCl3 is a highly reactive reagent, it is common practice to cool the mixture in an ice bath to compensate for any exotherms and to prevent a runaway reaction or fire (pre-cooling is standard procedure whenever highly reactive materials are mixed). {end-tooltip} The reactants and product do not absorb the radiation produced by our UV lamps. Therefore, you will need to use the same chemical “dip” reagent used in previous experiments to detect these compounds. When the reaction is complete, dilute the mixture with ether. Wash the mixture with 10% aqueous Na2SO4, dry, and concentrate by distillation. The residue will be used without further purification in the next reaction.
Weeks 2/3: Wittig Reaction & Workup, GC-MS, Modeling
Conduct all operations in a fume hood.
Place a stir bar in a small round bottom flask. Dry the apparatus with a heat gun (FIRE/BURN hazard {tooltip}[NOTE]{end-texte}The heat gun gets extremely hot. Always hold it by its handle (never grab the nozzle). Remove all solvents and flammable reagents from the hood before using the gun (your apparatus should not contain any compounds at this point).{end-tooltip}) and allow it to cool. Place methyltriphenylphosphonium bromide-sodiumamide (“instant ylide”, approx. 250 mg {tooltip}[NOTE]{end-texte}A pre-weighed sample of “instant ylide” will be provided to you.{end-tooltip}) in the cool flask. Add dry THF (1 mL) to the reagent and stir the mixture at 25oC for 15 minutes {tooltip}[NOTE]{end-texte}Adding THF to the phosphonium salt and base allows them to mix and react with each other. The result is a yellow ylide-containing solution and NaBr.{end-tooltip}.
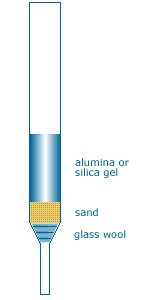
Pass 4-acetyl-1-methylcyclohexene through a microcolumn (alumina, dry THF {tooltip}[NOTE]{end-text}A microcolumn is constructed from a disposable pipet, glass wool, sand, and a powdered stationary phase. Use alumina for the stationary phase in this experiment.
Place a small amount of clean glass wool in a clean, empty pipet and poke it down to the narrow end. Next, add a small amount of sand on top of the wool, followed by the stationary phase (powder) on top of the sand (wool and sand are needed to keep the powder from falling out of the pipet). It is necessary to use pressure to force a liquid through the microcolumn. Attach a rubber pipet bulb to the column, squeeze the bulb to force the liquid into the column, remove the bulb from the column while still squeezing it, then release the bulb. Repeat the attach-squeeze-remove-release sequence as needed.{end-tooltip}) directly into the yellow ylide solution. Attach a reflux condenser fitted with a drying tube to the flask and reflux the solution for 20 minutes. Use TLC to confirm the presence of limonene in the reaction mixture {tooltip}[NOTE]{end-texte}The reactants and product do not absorb the radiation produced by our UV lamps. Therefore, you will need to use the same chemical “dip” reagent used in previous experiments.{end-tooltip}.
Work up the reaction by diluting the mixture with hexane (30 mL), washing with 10% aqueous K2CO3 (5 mL), and drying. Concentrate the organic layer to 2-3 mL by carefully and gently boiling the mixture on a hot plate. Finally, pass the residue through a fresh microcolumn (silica gel, hexane). Collect two or three ~2 mL fractions and use TLC to test them for limonene. Analyze the fraction that appears to contain the most limonene by GC-MS {tooltip}[NOTE]{end-texte}The GC-MS instrument is located in the sub-basement (first floor). Instructions for using the instrument will be provided in the instrument lab.{end-tooltip}.
Molecular Modeling
The molecules in this exercise exist as a mixture of conformers. The following instructions are designed to lead you to a specific conformer of each molecule. Please pay close attention to the figures that are provided so that you produce useful models.
Building the desired conformer. As you know, there are two ways to generate models in Spartan’18: sketching and building. When you sketch a molecule in a particular conformation, you often get a 3-D model in the same conformation. However, even if the 3-D model has a different conformation, you can always adjust the conformation to be the one you want.
Generate the 3-D model and select the Edit Build icon (or select Build: Edit Build). Click on the bond that you want to rotate (a red arrow will encircle the bond). Next, place the cursor in the shaded rotation zone on the left side of the screen (the top of the zone is labeled with a curly red arrow), press the LEFT mouse button and slide the cursor up and down in the shaded rotation zone.
Isoprene. Use Spartan’18 (Chemistry computer lab) to sketch or build a model of isoprene in the conformation shown below {tooltip}[NOTE]{end-texte}If you are building, select Groups: Alkenyl to build the diene. Then select sp3 C to add the methyl group.{end-tooltip}.
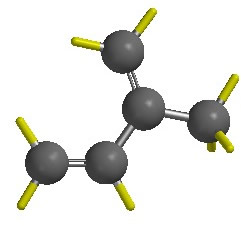
Select the Minimize icon (or select Build: Minimize) {tooltip}[NOTE]{end-texte}The picture in the lab manual shows isoprene with a planar pi system, but your model may not be planar. Small deviations from planarity do not affect MO energies much so don’t worry about this.{end-tooltip}. Click on Calculations… from the Setup menu. Set up the top menus to read: “Calculate: Equilibrium Geometry at Ground state with Semi-Empirical AM1.” Click on Submit.
After the calculation is complete, select Display: Properties. The energies of the HOMO and LUMO (in eV) will be displayed in the molecule tab. Record both MO energies.
MVK. Build a model of methyl vinyl ketone in the conformation shown below {tooltip}[NOTE]{end-texte}Select Groups: Alkenyl to build ethylene. Select Groups: Carbonyl to add the carbonyl group. Finally, select sp3 C to add the methyl group.{end-tooltip}.
Follow the “isoprene” procedure to minimize the MVK model, calculate its AM1 equilibrium geometry, and display its HOMO and LUMO energies. Record these energies.
MVK-AlCl3. Build a model of the methyl vinyl ketone-aluminum chloride adduct in the conformation shown below. Unfortunately, you won’t be able to sketch this molecule from scratch (sketch bug: Al is currently undefined), but you can generate this Lewis acid-base model using the indirect sketch+edit build method described in the next paragraph.

Sketch MVK-SiCl3. Notice that the sketch automatically adds a “+” charge to O when you draw a third bond to this atom. Convert your sketch into a 3-D model. Select the Edit Build icon (or Build: Edit Build). Adjust the conformation so that it looks like the figure shown above. Next, click on the Inorganic button (right side of window), click on whatever element name is displayed on the right side of the window (usually C (6) Carbon) and select Al from the miniature periodic table that pops up. Finally, double-click on the silicon atom in your model to replace this atom with aluminum.
Once you have built the model in the desired geometry, follow the “isoprene” procedure to minimize the model, calculate its AM1 equilibrium geometry and MO energies, and display its frontier MO energies. N.B. Be sure to change the overall charge back to neutral in the calculation set up window!
Important points.
1. Compare the LUMO energies of MVK and MVK-AlCl3. Which dienophile should be more reactive towards isoprene?
2. The Background section stated that the MO energies of most Diels-Alder reagents are consistent with the assumption: diene = Nu and dienophile = El.Are the HOMO and LUMO energies that you have obtained consistent with this assumption? Describe your results and how you used them to reach your answer.

