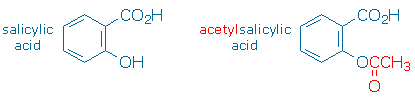Synthesis of Salicylic Acid from Oil of Wintergreen
Background
Methyl salicylate and salicylic acid are naturally occurring compounds with medicinal properties. On top of that, the procedure that you will apply here, saponification, is a well-known method for converting natural fats into soap. So is there some practical connection between making medicines and making soap? None that I know of.
But, let’s think about this in the way organic chemists do. Fats are esters of carboxylic acids, and soaps are the sodium salts of the corresponding carboxylic acids. Is there something special about fats that make these esters susceptible to saponification? If we answer in the negative (“No, I don’t think there is anything special about esters from fats”), then a natural way to test our hypothesis is to attempt the saponification of an ester that does not come from fats. That is exactly what we will do here.
The rest of this page looks more closely at saponification from the standpoint of functional groups (carboxylic acids, esters, etc.), and chemical reaction mechanisms, but first, I can’t resist sharing some stories about the medicinal uses and history of these remarkable compounds.
Salicylic acid
Salicylic acid is produced by a range of plants and many societies made use of these plants as primitive medicines. Willow trees, in particular, have been recognized since antiquity as a potent source of medicine. Hippocrates encouraged the pregnant women of ancient Greece to chew willow leaves and drink willow tea to alleviate labor and birth pains. This knowledge was not limited to the Mediterranean either. Ancient societies around the globe from China to the New World placed a high value on the medicinal properties of willow.
Salicylic acid is most abundant in willow leaves and bark. The medicinal properties of salicylic acid include being an antipyretic (reduces fever), an analgesic (reduces pain), and an anti-inflammatory (reduces swelling). Interestingly, these same three properties are also displayed by a structurally related compound, acetylsalicylic acid, more commonly known as aspirin. Aspirin does not occur naturally. It must be manufactured in the laboratory. So, given two compounds with similar medicinal properties, one naturally occurring and the other available only through chemical synthesis, why go to all the trouble of making and taking aspirin? Why not just chew willow leaves or drink willow tea?
The problem with pure salicylic acid, and even willow, which contains a diluted version of salicylic acid, is that these substances are hard for the body to tolerate. The salicylic acid molecule contains two acidic functional groups, the phenol group (ArOH) and the carboxylic acid group (-CO2H). These chemically reactive groups make salicylic acid an irritant. When it comes into contact with the sensitive linings of the mouth, throat, esophagus, and stomach, it burns these tissues. (Interestingly, salicylic acid’s ability to burn living tissue has its uses: salicylic acid is the active ingredient in Compound W, an over-the-counter wart remover.)
Aspirin
At the end of the 19th century chemists discovered that they could mitigate salicylic acid’s harsher qualities by replacing one of its acidic hydrogens with a less reactive group of atoms. Aspirin (acetylsalicylic acid) replaces the phenolic hydrogen with an acetyl group, -COCH3. Once it enters the bloodstream, acetylsalicylic acid quickly reacts with water to regenerate salicylic acid and acetic acid.
Aspirin is a fascinating substance for many reasons, but the story of its discovery is especially revealing. I don’t know what an online search on aspirin would produce today, but in the early days of the web (= 1990’s) I discovered that I could learn a lot via searches that combined “aspirin” with other keywords like “discovery,” “patent,” and so on. Here are two interesting stories that I stumbled upon:
- Friedrich Bayer & Co., the first company to test aspirin as a commercial product, did not allow its release initially because the head of Bayer’s pharmacology lab was convinced that aspirin would damage the human heart (this person had a clause in his contract that allowed him to blackball any compound that he didn’t trust). Many years later it was discovered that a daily aspirin pill actually helped prevent heart attacks. Science advances through the mistakes of scientists.
- Bayer was forced to relinquish its aspirin patent as part of the Treaty of Versailles following World War I. This action was demanded by the Allies as part of the war reparations.
Probably the most remarkable story I found concerned the discovery of aspirin. The discovery story that I had been taught as a student in the early 70’s (and that I subsequently taught to my students) went like this: Felix Hoffmann, a young Bayer chemist, had been encouraged by his ailing father to look for compounds that would alleviate the father’s arthritis pains. The result was aspirin, which Hoffmann first prepared and gave to his father in 1897. In some versions of the story, Hoffmann’s decision to make aspirin is described as an accident, while in others, his decision is described in a more intentional way: he had heard or read about the medicinal properties of impure aspirin and decided to make a pure sample for testing.
Even if we don’t know why Hoffmann decided to make aspirin, his sick father and the lucky discovery all make for a good story, which makes it rather remarkable that this story was apparently suppressed (?) for 37 years. The first time any version of the Hoffmann discovery appeared in print was in 1934 when it appeared as a a footnote in a German book describing the history of chemical engineering.
Perhaps even more remarkable was another story told about aspirin’s discovery that was printed shortly after World War II. In this version, a Jewish colleague of Hoffmann’s in the Bayer laboratories, Dr. Arthur Eichengruen, tells how he had planned and directed the synthesis of aspirin (along with several related compounds), and how he had arranged for aspirin’s initial surreptitious clinical testing (apparently the head of the Bayer pharmacology lab was opposed to clinical testing). According to Dr. Eichengruen, Hoffmann participated only in the initial laboratory synthesis of aspirin and nothing more. In fact, Hoffmann may not have even known why he was making aspirin.
It’s rather curious that the Eichengruen version was available in the late 1940’s, long before I began studying organic chemistry in 1973, and yet I totally unaware of it. Why? Partly because it was denied by the Bayer company, and subsequently ignored by historians and chemists. Our understanding of the true story of aspirin’s discovery began to change only in 1999, when another scientist, Walter Sneader, re-examined all of the documents involved and published his detailed analysis of the historical record (BMJ, 2000, 321, 1591). According to Sneader, the “original” 1934 footnote did not fit the known facts about aspirin, whereas Eichengruen’s “new” account was convincing on all counts.
You might wonder how such a fundamental and important discovery could have been the subject of a Big Lie for so long, but an explanation is not that hard to construct. German life was controlled by the Nazi party in 1934. The party had taken many steps to remove Jews from many professions, and they had also taken it upon themselves to rewrite German history (to “Aryanize” it). This included rewriting German scientific history. Of course, contradicting Nazi propaganda at that point in time would have been a one-way ticket to oblivion for a Jewish chemist. Eichengruen had to wait until the war was over, and his safe release from the Theresienstadt concentration camp, before he could finally tell the true story. I am sad to say that when I first inserted this account into our lab manual (Aug. 2003), the Bayer company was still refusing to acknowledge the contributions of Dr. Eichengruen.
Methyl salicylate
Methyl salicylate, like salicylic acid, is found in many plants. It was first isolated from wintergreen leaves, Gaultheria procumbens, and is often called oil of wintergreen.
Methyl salicylate is similar to acetylsalicylic acid in that it masks one of the acidic hydrogens in salicylic acid, however, methyl salicylate replaces the carboxylic acid hydrogen with a methyl group, -CH3. The result is a relatively unreactive compound that does not liberate salicylic acid efficiently in the body. Consequently, methyl salicylate is not useful in any of the ways aspirin is.
On the other hand, methyl salicylate has other uses, mostly based upon its distinctive fragrance. It can be found in many commercial products, including root beer and Ben-Gay ointment.
Saponification
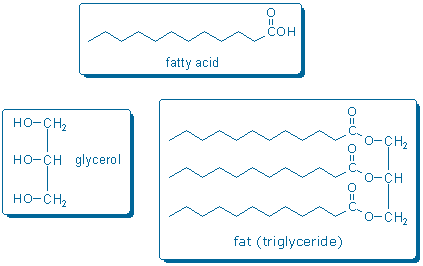
Natural fats are called triglycerides because they contain glycerol and three fatty acids. The functional group that links glycerol with each fatty acid is called a carboxylic acid ester. Thus, a triglyceride contains three ester groups. (Sorrell pp. 702-4 describes how to name carboxylic acids and related compounds).
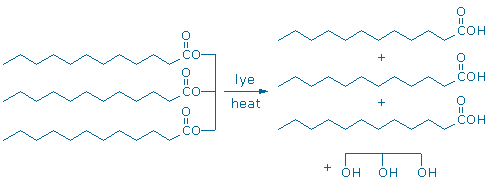
Fats can be converted into fatty acids and glycerol by boiling fat with lye (aqueous NaOH). This process is called saponification (Sorrell pp. 716-7) because the products make a nice soap. Notice that the chemical equation drawn below is not balanced; more atoms are needed on the reactant side of the equation (lye supplies the missing atoms).
A completely analogous process converts methyl salicylate into salicylic acid. Methyl salicylate is a carboxylic acid ester too, and it reacts with lye to make a carboxylic acid (salicylic acid) and an alcohol (methanol).
The analogous chemical behavior of “triglyceride +lye” and “methyl salicylate + lye” illustrates a widespread chemical phenomenon: functional groups, like carboxylic acid esters, react in pretty much the same way regardless of their molecular surroundings. This principle, which we might call the “functional group principle”is probably the most important conceptual tool in all of organic chemistry.When an organic chemist looks at the formula of an unfamiliar compound, the first thing they do is look for familiar functional groups.
Saponification reactions will be studied in detail during Chemistry 202, so only a few points will be mentioned here.
- The saponification reaction occurs in several mechanistic steps. These include:
- Addition of hydroxide ion to the carbonyl group to give a “tetrahedral intermediate” (Sorrell, p. 717, step 1). The “tetrahedral intermediate” is given this name because the carbonyl carbon briefly adopts a tetrahedral geometry.
- Dissociation of methoxide ion, CH3O–, from the tetrahedral intermediate to form the carboxylic acid + CH3O–.
- A fast proton transfer between the carboxylic acid and unreacted HO– (or CH3O–) to give the conjugate base of the carboxylic acid and H2O (or CH3OH) (Sorrell, p. 717, step 3).
- If you have followed this description with an open copy of Sorrell, you will notice that I didn’t mention step 2 (p. 717). I view step 2 as a controversial drawing of what is occurring and I would encourage you not to learn it for now. Sorrell describes step 2 with these words, “assistance is provided by the aqueous solvent, which can transfer a proton to the base (CH3O–) as it leaves.” This description may be accurate, but if it is, it raises a question about the mechanism of the reverse reaction. The reverse of step 2 would involve three molecules — CH3OH, HO–, and the carboxylic acid — and it would demand that they all collide simultaneously with just the right geometry. This seems highly unlikely. Also, CH3OH is a slightly stronger acid than H2O so it seems unreasonable to imagine it becoming (or staying) protonated in an aqueous NaOH solution.
- The saponification of methyl salicylate is even more complicated than that of normal esters. Methyl salicylate contains an acidic phenol group (PhOH). This group reacts on contact with aqueous lye to give an insoluble white goo. The ions in this goo subsequently undergo saponification a la Sorrell (and the goo dissolves as the saponification proceeds). So just be aware that the ester that is being attacked by HO– already contains a negatively charged group elsewhere in the molecule. This matter is discussed further in the Procedure).
- Finally, realize that this reaction mixture is basic to begin with (it contains HO–), and stays basic throughout (the final mixture contains HO–, CH3O–, and the conjugate base of salicylic acid). In order to obtain a neutral carboxylic acid, we must “work up” this reaction mixture by adding an acid that is strong enough to react with all of these bases (they will be converted into H2O, CH3OH, and salicylic acid, respectively). Favorable acid-base reactions like this are exothermic and the solutions that contain reacting acids and bases can grow quite hot. For this reason, you must cool the reaction mixture before you add acid to it and you must add the acid in small portions.
Continue to Procedure…