Synthesis of Salicylic Acid from Oil of Wintergreen
Procedure
Pre-lab preparation
First, prepare your notebook. Starting a new (right-hand!) page, do the following in the sequence listed:
- write a descriptive title for the experiment (“Preparation of Salicylic Acid from Methyl Salicylate”, “Saponification of Methyl Salicylate” are both good options)
- draw a chemical equation describing the transformation that is expected. The drawing should show methyl salicylate + NaOH as reactants, and salicylic acid + methanol as products. By definition, an “equation” must be balanced. The “equation” described here is not balanced, but it is probably more useful because it shows the compounds that you will be consuming and producing in the forms that you will encounter. To put it another way, this drawing serves as a practical description of what the experiment is about.
- construct a table of physical properties. The table should include separate columns for compound/reagent label, MW, mp, bp, d, and for “amount desired” (see #6 below), and separate rows for each compound/reagent that you will handle in this experiment (methyl salicylate, salicylic acid, methanol, 6 M aqueous NaOH, and 3 M aqueous H2SO4. It is not necessary to fill in all of the entries for every reagent. List the concentrations of the NaOH and H2SO4 solutions, not their MW. Likewise, list the densities of these reagents, but not their mp or bp.
- list any unusual hazards that a lab worker should be aware of along with safety protocols that address these hazards
- list disposal information for all compounds. Aqueous solutions should never be placed in the organic waste containers. Acidic and basic solutions must be partially neutralized before they can be sent down the sink. A small amount of methanol is produced in an aqueous solution and it is impractical to separate the methanol from the water so both will be sent down the sink together. A special container will be provided to collect salicylic acid at the end of the experiment.
- calculate the amounts of reagents that you expect to use (you can use your left notebook page for calculations, but your and/or under a column called “amount desired” in your table – make sure you list the units)
- (left-hand page) write the entire procedure (with amounts) that you will follow. To save time and space, feel free to use meaningful (!) abbreviations as appropriate, and to include only information (operations + warnings) needed to complete the experiment safely. Drawings of apparatus may also be useful here.
Safety notice: Wear gloves and goggles at all times and carry out all operations in a fume hood. While goggles + fume hood are standard operating procedures in our lab, gloves are required for the handling of strong bases and acids.
Online videos. Videos of the procedure are available through the Moodle or you may want to review the following videos on lab procedures before lab (these are all from the Royal Society of Chemistry’s Interactive Lab Primer):
- Simple Reflux (full video)
- Buchner (vacuum) Filtration (skip dessicator)
- Recrystallization (full video, but no ‘hot’ filtration for us)
Week 1: Reaction
Equipment
- 50 mL round bottom flask
- lab jack + stirring motor
- magnetic stir bar
- ceramic heater + controller
- graduated cylinder
- screw clamps
- reflux condenser & water hoses
- funnel
- disposable pipettes
Procedure
In your fume hood: place a stirring motor on top of a partially opened lab jack, then place a heater on top of the motor. Clamp the round bottom flask to the monkey bars in your fume hood so that it rests in the heater. Plug the heater into the controller, but do not turn on the controller (never plug a heater directly into a wall socket).
Transfer sufficient 6 M aqueous NaOH into a graduated cylinder so that you have 120 mmol of NaOH, then transfer this liquid to the round bottom flask using a funnel. Liquid reagents are most conveniently measured by volume. You must calculate the desired volume of reagent before making any measurements (see Calculations for help). You must also record the actual volume used in your lab notebook. It is not necessary to hit the desired amounts precisely, but you should record your measurements precisely.
Rinse your funnel and graduated cylinder with water to remove all traces of the reagent, and remove any stray drops of reagent from the ground-glass neck of your flask. Aqueous NaOH reacts with the compounds that make up glass, etching and dissolving the glass. Two pieces of glassware can even “freeze” into a single unit if aqueous NaOH is present on the surface where the two pieces touch. Therefore, you should always remove this reagent from ground glass surfaces before assembling apparatus.
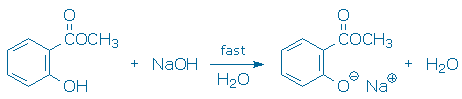
Transfer about 21 mmol (see Calculations for help) of methyl salicylate into a graduated cylinder then add it to the round bottom flask using a funnel. You should see a white gummy material form in your flask. Methyl salicylate reacts instantly with NaOH to make a white gummy material, but this does not hinder the eventual formation of salicylic acid. The white gummy material may be the product of an acid-base reaction.
Equip the flask with a stir bar, reflux condenser, and water hoses. Begin stirring. Slowly flow water through the reflux condenser and adjust the controller voltage to initiate reflux. Reflux the mixture for 10 minutes, while stirring as best you can (the gummy material prevents the stir bar from working at first, but the material soon dissolves). Turn off the controller and remove the lab jack, motor, and heater. Allow the mixture to cool to room temperature before continuing. Your glassware is made out of Pyrex and should not break if subjected to modest temperature shocks. You can take advantage of this fact to quickly cool the reaction mixture by immersing the flask in a large beaker of water.
Week 1: Work-up
Equipment
- 150 mL beaker
- pH paper
- 400 mL beaker (for ice water bath)
- 250 mL filtering flask
- heavy-walled rubber hose
- Buchner funnel + neoprene adaptor
- filter paper
Procedure
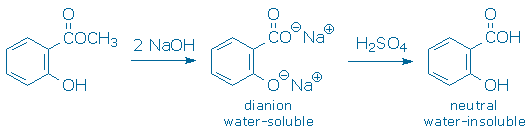
Pour the cooled mixture into a 150 mL beaker. Add sufficient 3 M aqueous H2SO4 to precipitate all of the salicylic acid. The reaction between methyl salicylate and NaOH produces the dianion of salicylic acid. This dianion, because of its electrical charge, is water-soluble. It is also a weak base, so it reacts with strong acids, like aqueous H2SO4, to give neutral, water-insoluble salicylic acid. You do not need to calculate how much acid to use. Rather, you should add acid until the solution’s pH (measured with pH paper) is sufficiently below the pKa of salicylic acid to guarantee that nearly all of salicylic acid has been neutralized. The pKa of salicylic acid is approx. 3, so any pH of approx. 1 or less is satisfactory.
Place the beaker in an ice-water bath for several minutes, then collect your product by vacuum filtration. While still applying a vacuum, rinse the collected solids with a small amount of ice-cold water and let them air-dry for several minutes with the vacuum on. Salicylic acid precipitates from a solution containing a large number of impurities: Na+ and SO42- ions and methanol molecules. Some of these impurities get trapped in the solid, but others can be removed by rinsing with water. Use small amounts of ice-cold water to minimize loss of salicylic acid.
Week 1 : Recrystallization
See Padias pp. 121-129 for information about recrystallization.
Equipment
- Two 250 mL Erlenmeyer flasks
- boiling chips
- hot plate (stirring motor)
- disposable pipets + rubber bulbs
Procedure
Place your crude salicylic acid and 5-10 mL of deionized water in a 250 mL Erlenmeyer flask (“solids” flask). Place about 80-120 mL of deionized water in a second flask (“solvent” flask). Add 2-3 boiling chips to each flask. Place the “solvent” flask on a hot plate and bring the water to a boil. Next, set the “solids” flask next to the “solvent” flask on the hot plate and bring the “solids” mixture to a boil as well. Transfer just enough boiling water from the “solvent” flask to the “solids” flask to dissolve all of the solids in the “solids” flask.
Recrystallization separates a product from impurities by 1) dissolving everything in hot solvent and 2) cooling the resulting solution. If all goes well, the desired product crystallizes as the temperature falls, but the impurities (now present in relatively small amounts) do not. Unfortunately, some product is always lost during recrystallization because some remains dissolved in the cold solution. Therefore, the “trick” for a successful recrystallization is to use just enough solvent to get everything to dissolve, but no more than that. Here are some common mistakes that you can avoid. 1) Never remove the “solids” flask from the hot plate to examine it; the solution will cool and the product will crystallize. 2) Solids do not dissolve instantly, but do not wait too long for them to dissolve because the solvent will boil away while you wait. 3) It is better to use slightly too much solvent (and lose some of your product) than too little (and fail to separate the impurities from your product).
Remove the “solids” flask from the hot plate and let the solution cool slowly. Salicylic acid should crystallize spontaneously. Watch closely – it is a fascinating process. After the solution has cooled sufficiently, recover the product by vacuum filtration (use a clean filter flask and funnel). Set the product on a labeled watch glass underneath a hood to air dry.
Week 2: Characterization
Although your crystals should now be quite pure, they are coated with water. Water molecules may even be embedded in the crystals. Characterization should not begin until you have removed as much of this water as possible. You will rely on air-drying to evaporate residual water, a slow process that requires at least 24 hours. Most students will be able to let their product air-dry for a week before characterizing it during the next lab session.
Record the weight of your dry product and its melting temperature. See Padias pp. 47-53 for information about characterization procedures, including accepted sources of scientific data, phase diagrams, and melting point measurements. Several different apparatuses are available for use. Safety tip: every melting apparatus contains a heater that can ignite; turn off the apparatus after use. Remember that 2-3 trials are needed, and that the data needed from each trial includes two temperatures, the temperature at which solid-to-liquid conversion first occurs, and the temperature at which the last bit of solid changes into a liquid. In addition to the melting point, prepare an NMR sample (dissolve approximately 10-20 mg in 0.5 mL of CDCl3) and take an FT-IR (ATR) of your final product. Instructions for how to take an IR of a solid will be provided in lab.
Continue to Report…