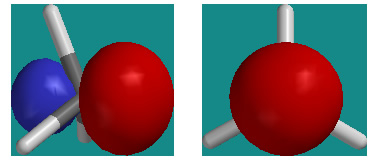
Today’s lecture on conformational isomers and internal rotation omitted a rather slick use of hybrid orbitals. Pictured above are two views of CH3 (methyl) with its singly occupied sp3 hybrid orbital on C (side view on left, orbital axis view on right). Notice the cylindrical symmetry of the orbital about the orbital axis.
Now imagine CH3 were to bond to another CH3 to make ethane, C2H6. Both fragments would overlap their sp3 orbitals along their symmetry axes to make a bonding MO occupied by two electrons. Since both hybrid orbitals have cylindrical symmetry, they would overlap (and stabilize the electron pair) in exactly the same way for both a staggered and eclipsed conformer.
This helps explain why conformers are so close in energy. Internal rotation can occur without weakening the bond that the groups rotate around.