Ferrocene and acetylferrocene, the compounds featured in our current lab experiment, are examples of sandwich compounds or metallocenes. A metallocene of some sort has been made with every transition metal in existence, but double metallocenes in which two organic rings are fused together (see figure) have proved more elusive. The first ones were made in the 1970s, but chemists couldn’t figure out how to fit very many metals into the double metallocene structure.
A recent article in the Journal of the American Chemical Society (J. Am. Chem. Soc., 2008, 130 (46), pp 15662-15677, web publication Oct 22, 2008) describes how chemists have solved these problems to make double metallocenes of V, Cr, Mn, Co, and Ni. Some of these complexes (V, Mn) seem to contain direct metal-metal bonds, while others (Cr, Co, Ni) do not.
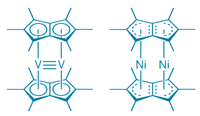
C&E News, Nov 3, 2008, p. 22
Curiously, all attempts to make a double ferrocene were unsuccessful, so while ferrocene occupies a privileged place as the first metallocene to be made, double ferrocene may prove to be an impossible target. But who knows? Chemists love a challenge.