The slides I showed in lecture today are available here.
I have three short comments to go with these:
Continue reading
The slides I showed in lecture today are available here.
I have three short comments to go with these:
Continue reading
I thought I was setting the record straight on energy yesterday, but upon reflection, I made an egregious mistake. Well, I probably made several, but there’s only one that I’m currently aware of.
In drawing a distinction between free energy (G) and enthalpy (H), I unintentionally conflated enthalpy with potential energy. Fortunately, the situation is easily corrected.
Continue reading
On p. 146 Loudon asks “why an sp2-sp3 C-C bond is stronger than an sp3-sp3 C-C bond”. It’s a good question because it might help explain why more substituted alkenes are more stable than their less substituted isomers, a topic that was covered in today’s lecture. Unfortunately, the explanation provided by Loudon strikes me as incorrect.
Continue reading
Chapter 4 introduces so-called “road map” problems. Problem 4.51 & 4.52 are typical examples. A “road map” problem gives you some information about the composition of a molecule and its chemical behavior (what you can turn it into, how you can make it from other substances). Your job is to figure out the molecule’s structure.
A key step in the solution of a “road map” problem is to calculate the molecule’s unsaturation number (also called “degree of unsaturation” and “number of units of unsaturation”). Loudon explains how to perform this calculation in section 4.3 and I won’t cover this material in lecture. I do want to call your attention, however, to an error in the book. The term “2C” should appear only once in equation 4.7 (compare equations 4.5, 4.6, and 4.7).
FYI – I learned a slightly different (but mathematically equivalent) equation as a student: U = #C + 1 – #H/2 + #N/2.
Whichever equation you learn, notice that:
Now that I’m finished reading lab notebooks, I want to share a couple of general observations. The first and most important one is this: the lab notebooks were generally good. Most of them were prepared well, used effectively in lab, and succeeded as records of scientific work. Keeping a good notebook may not seem like much of an achievement, but it took a fair amount of time for you to read all of my instructions, consult the examples in Padias, look up data on compounds, and so on, so you have deservedly earned my praise. Well done.
Of course, I also saw some gaps here and there, but only three deserve special comment (I promise to be brief).
Continue reading
Slides for today’s lecture are located here. The table of common acidic functional groups and related properties are located here (note: you need to memorize the information in this table for next Friday’s exam + future exams). The table will also be included with the study guide for chapter 3 whenever that gets posted (hoping to have it ready in the next 24 hours).
The standard way to think about steric repulsion is very simple. An atom requires space for its electrons. If another atom intrudes on that space, both atoms are unhappy and the energy rises. Steric repulsion.
Unfortunately, concept doesn’t quite match reality here. It is so costly for atoms to “overlap” (unless they are bonded, of course) that they rarely do. For example the distance between the end C in butane, C-C-C-C, is practically the same in the relatively low energy gauche conformation (3.1 A) and for the very high energy “methyls eclipsed” conformation (2.9 A). (For comparison, the distance is about 3.8 A in the anti conformation.) We know that the “methyls eclipsed” conformation is destabilized by steric repulsion, but the distance between the methyl groups is still fairly large. What’s going on?
Continue reading
Loudon mentions two van der Waals forces in Chapter 2, an attractive force and a repulsive force. This is confusing because, other than the fact that both forces have something to do with electrons, they are not related.
The attractive van der Waals force is described nicely in Figure 2.8. It appears when the distance between two atoms (or molecules) is short, but not too short (if the atoms’ space-filling models are just touching or are separated by a small gap, then that’s perfect). The force is created by temporary imbalances in each atom’s electron distribution caused by electron movement around the nucleus. The imbalances produce small temporary electrostatic fields and two atoms will naturally correlate their imbalances (fields) so that they attract one another. The attractive force is very weak.
The repulsive force is another beast entirely. It suddenly replaces the attractive force when the distance between the atoms is less than the sum of the atoms’ van der Waals radii. The repulsive force is very strong and we blame it on Pauli repulsion, the tendency of same-spin electrons to avoid each other (or “die” trying).
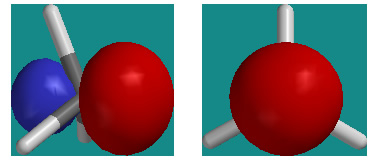
Today’s lecture on conformational isomers and internal rotation omitted a rather slick use of hybrid orbitals. Pictured above are two views of CH3 (methyl) with its singly occupied sp3 hybrid orbital on C (side view on left, orbital axis view on right). Notice the cylindrical symmetry of the orbital about the orbital axis.
Now imagine CH3 were to bond to another CH3 to make ethane, C2H6. Both fragments would overlap their sp3 orbitals along their symmetry axes to make a bonding MO occupied by two electrons. Since both hybrid orbitals have cylindrical symmetry, they would overlap (and stabilize the electron pair) in exactly the same way for both a staggered and eclipsed conformer.
This helps explain why conformers are so close in energy. Internal rotation can occur without weakening the bond that the groups rotate around.
I have read all of the assignments that were turned in and I will be returning them in class on Monday. In addition to the comments that my graders and I have put on your assignments, there are two additional things that I want to bring to your attention:
- To make grading go more quickly, my two graders and I have used a set of two- and three-letter codes. These were also used last year and you can find an explanation of these codes at this post from September 15, 2008.
- I made a mistake when writing my answers for HW #1. When I read Loudon 1.39 (problem #6), I mistakenly substituted HCl and HF for CH3Cl and CH3F, respectively. The central point of my answer, Cl makes longer bonds than F, still applies, but my comments about treating atoms as charged particles need to be modified. Come see me if you would like to discuss this or any other matter pertaining to the course.