This assignment seems to have set a new “standard” for
stumping students and I found myself writing “please come see me this week”
messages on an unprecedented number of assignments.
First, let me talk about the “come see me” note.
The point of visiting me is quite simple – as your instructor (“study coach”),
I want to understand as much as I can about difficulties that you are having in
my course. Some difficulties are an inevitable part of the learning process.
Others might be avoided, or moderated, by engaging in different study
practices. By talking about this together, I might be able to suggest some places
where your approach can be improved. So please follow up asap on my request to “come
see me”. (And if your homework doesn’t contain such a note, feel free to come
see me. I like the company.)Now, about the homework problems themselves. The answers have been posted and I encourage every student to look at every answer even if
we wrote “good” or “nice” next to your answer. It is always helpful to see how
another person (especially the instructor) states the answer to a problem.
Please look out for, and try to understand, all differences between your answer and the posted answer. Here are
some areas where these differences really stood out:
Number CH and CC BMO matches bond order. If C and H, or C and C, atoms are joined by a single bond then this bond is created by an occupied BMO (always sigma). If two C are joined by a double bond then this bond is created by two occupied BMO (one sigma and one pi).
Reaction energy
diagrams. Does your diagram contain a transition state for every
mechanistic step? (Two steps/transition states are needed.) Does your diagram
contain the correct species at each point? (A common mistake: leaving out Br-
or Cl- in the intermediates.) Do the energy curves rise and fall by an
appropriate amount? Do slower reactions have larger barriers in the
rate-determining step?
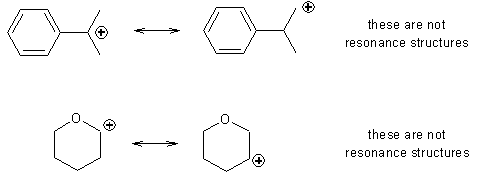
Failing to draw
resonance structures correctly. Failing to recognize the significance of
resonance structures. If the charge in a carbocation is delocalized, that
cation is resonance-stabilized. It will form more rapidly. If the charge is not
delocalized, no resonance-stabilization exists, and the cation will form more
slowly. Be very, very, very careful when using skeletal formulas to represent
resonance structures. The following drawings show the kinds of errors that skeletal formulas lead to (if you add the correct
number of H to each C, you will see that the so-called resonance structures
actually have one H in a different location.)
Hydrogen-bonded
molecules. The most common problem was to invoke a geometry that cannot
possibly bring both hydrogen bonds into existence. The preferred geometry is a
linear arrangement of the three atoms in D-H…A. If two molecules form two
hydrogen bonds, these will need to involve parallel D-H…A groups. Another common
problem: drawing the wrong structural formula for adenine and/or thymine. When
a problem uses a compound’s name without providing a formula, look up the
correct formula.
Drawing reagents. Lots of o-chem problems will ask you to fill in missing reagents for a synthetic step. Let’s agree on what counts as a “reagent”.
- One way to define “reagent” is this: it should be something that you can find on the label of a reagent bottle. In our lab, we have bottles labeled “H2O” and “H2SO4“. These are excellent reagents. We do not have bottles labeled “H3O+“. Therefore, the reagents that should be drawn for the hydration of an alkene are: H2O and H2SO4.
- Major loophole: if your book draws “X” as a reagent then I will accept it. “X” must appear as a reagent in a synthetic step; species that appear in mechanistic steps are not necessarily reagents. Since your book draws “NaBH4, HO–” as reagents in chapter 5, I will accept “HO–” when combined with NaBH4 even though this goes against my first guidelines of “must be a bottle label”.
- Minor loophole: All guidelines may get relaxed in Chem 202. Growing sophistication has its privileges.
